19. When 0.75 moles chromium(III) oxide reacts with 2.5 moles hydrogen sulfide (H2S) gas, chromium(III) sulfide and water were formed. What is the percent yield of Cr2S3 if 125 grams of it was obtained? MW: Cr2S3 = 200.19g/mol Cr2O3(s) + 3H2S(g) -> Cr2S3(s) + 3H2O()
19. When 0.75 moles chromium(III) oxide reacts with 2.5 moles hydrogen sulfide (H2S) gas, chromium(III) sulfide and water were formed. What is the percent yield of Cr2S3 if 125 grams of it was obtained? MW: Cr2S3 = 200.19g/mol Cr2O3(s) + 3H2S(g) -> Cr2S3(s) + 3H2O()
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.70P
Related questions
Question
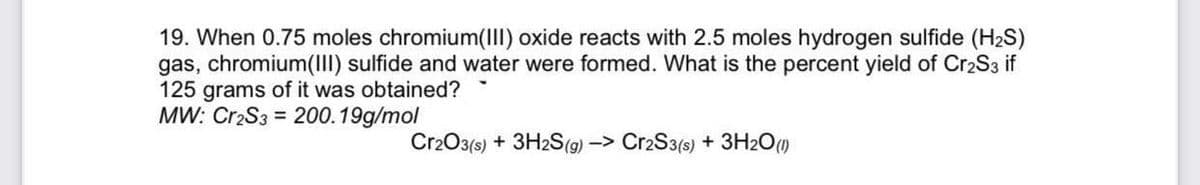
Transcribed Image Text:19. When 0.75 moles chromium(III) oxide reacts with 2.5 moles hydrogen sulfide (H2S)
gas, chromium(III) sulfide and water were formed. What is the percent yield of Cr2S3 if
125 grams of it was obtained?
MW: Cr2S3 = 200.19g/mol
Cr2O3(s) + 3H2S(g) -> Cr2S3(s) + 3H2O()
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning