Carbon dioxide (CO2) is the gas that is mainly responsible for global warming (the greenhouse effect). The burning of fossil fuels is a major cause of the increased concentration of CO2 in the atmosphere. Carbon dioxide is also the end product of metabolism. A general overall equation for this very complex process represents the degradation of glucose (C,H12O6) to carbon dioxide (CO2) and water (H20). C,H1206 + 602 → 6CO2 + 6H20 Using glucose as an example of food, calculate the annual human production of CO2 in grams, assuming that each person consumes 4.69 × 102 g of glucose per day. The world's population is 6.50 billion and there are 365 days in a year. x 10 Enter your answer in scientific notation.
Carbon dioxide (CO2) is the gas that is mainly responsible for global warming (the greenhouse effect). The burning of fossil fuels is a major cause of the increased concentration of CO2 in the atmosphere. Carbon dioxide is also the end product of metabolism. A general overall equation for this very complex process represents the degradation of glucose (C,H12O6) to carbon dioxide (CO2) and water (H20). C,H1206 + 602 → 6CO2 + 6H20 Using glucose as an example of food, calculate the annual human production of CO2 in grams, assuming that each person consumes 4.69 × 102 g of glucose per day. The world's population is 6.50 billion and there are 365 days in a year. x 10 Enter your answer in scientific notation.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
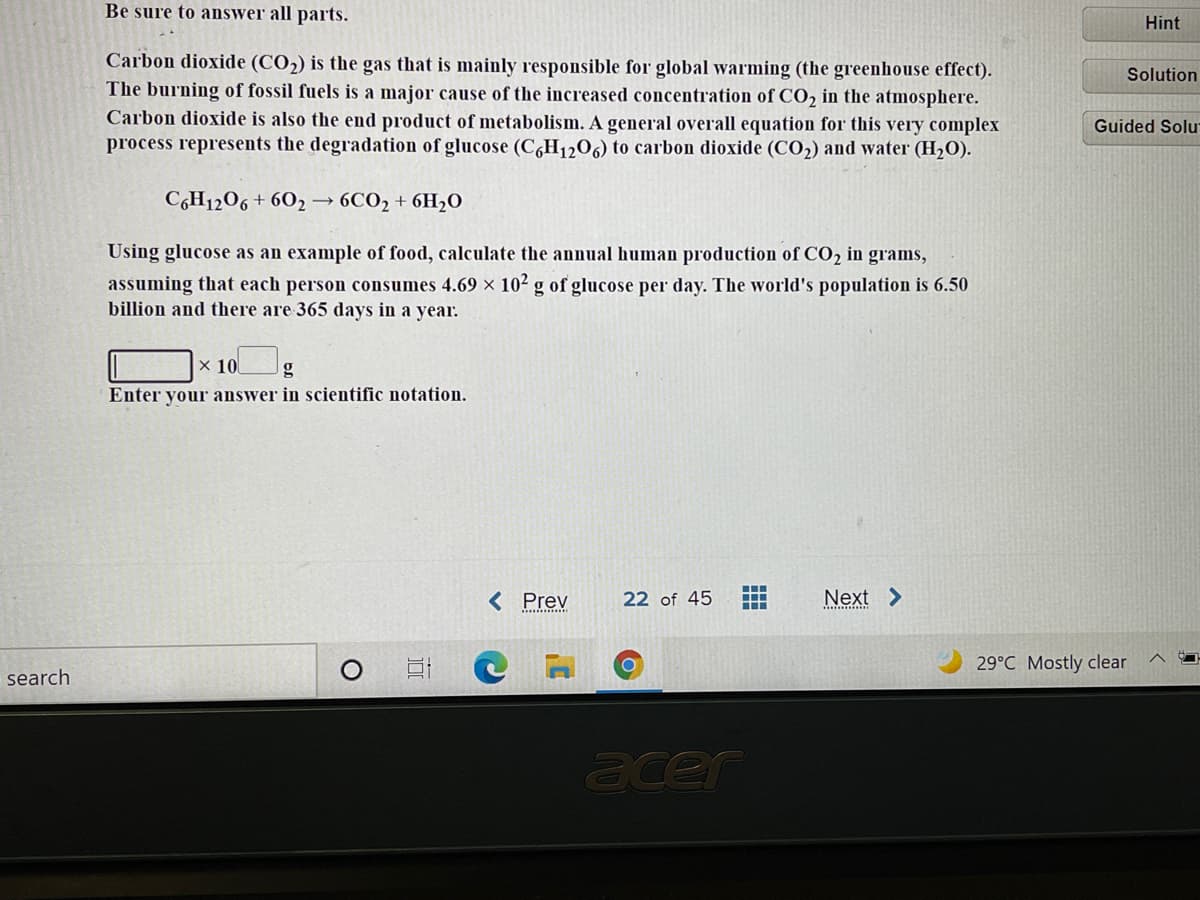
Transcribed Image Text:Be sure to answer all parts.
Hint
Carbon dioxide (CO2) is the gas that is mainly responsible for global warming (the greenhouse effect).
The burning of fossil fuels is a major cause of the increased concentration of CO, in the atmosphere.
Carbon dioxide is also the end product of metabolism. A general overall equation for this very complex
process represents the degradation of glucose (C,H1206) to carbon dioxide (CO2) and water (H2O).
Solution
Guided Solu
C6H1206 + 602→ 6CO2 + 6H,0
Using glucose as an example of food, calculate the annual human production of CO2 in grams,
assuming that each person consumes 4.69 x 102 g of glucose per day. The world's population is 6.50
billion and there are 365 days in a year.
x 10
g
Enter your answer in scientific notation.
< Prev
22 of 45
Next >
29°C Mostly clear
search
acer
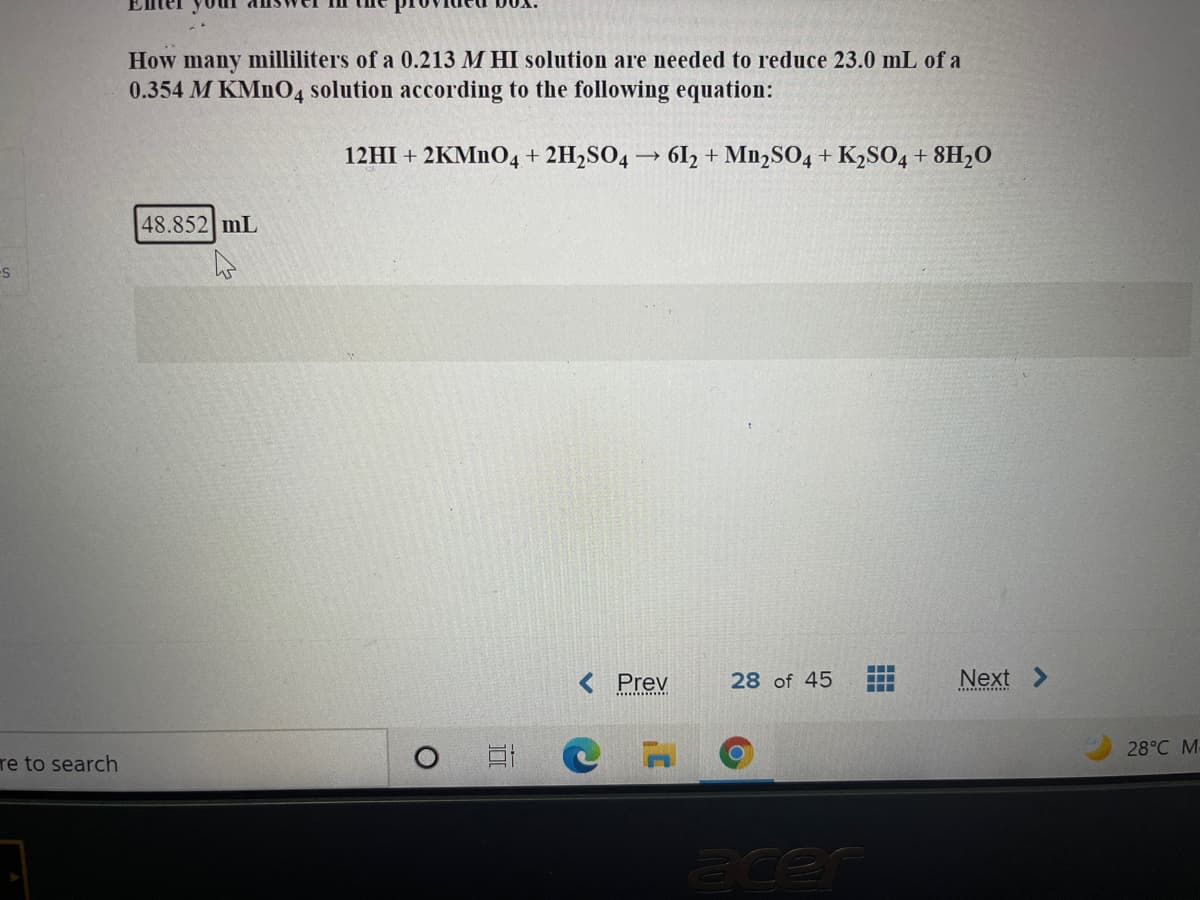
Transcribed Image Text:How many milliliters of a 0.213 M HI solution are needed to reduce 23.0 mL of a
0.354 M KMNO4 solution according to the following equation:
12HI + 2KMNO4 + 2H2SO4 → 61, + Mn,SO4 + K2SO04 + 8H2O
48.852 mL
Prev
28 of 45
Next
28°C M-
re to search
acen
田
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning