Carbon monoxide poisoning is a serious concern, even at low-levels of carbon monoxide in the air. Carbon monoxide preferentially replaces oxygen in oxygenated hemoglobin (Hb) according to the reaction: (bp)*0 + (bv)034H = (bn)0ɔ + (bp)*0qH Use the following reactions and associated equilibrium constants (at body temperature) to find the equilibrium constant for the reaction shown above. Hb(aq) + 02(aq) = HbO;(aq) K. = 1.8 Hb(aq) + CO(aq) = HBCO(aq) K = 306 Suppose that an air mixture becomes polluted with carbon monoxide at a level of 0.20%. Assuming the air contains 20.0% oxygen and that the oxygen and carbon monoxide ratios that dissolve in the blood are identical to the ratios in the air, what is the ratio of HBCO to HbO; in the bloodstream? Based on your calculations, just how toxic is carbon monoxide?
Carbon monoxide poisoning is a serious concern, even at low-levels of carbon monoxide in the air. Carbon monoxide preferentially replaces oxygen in oxygenated hemoglobin (Hb) according to the reaction: (bp)*0 + (bv)034H = (bn)0ɔ + (bp)*0qH Use the following reactions and associated equilibrium constants (at body temperature) to find the equilibrium constant for the reaction shown above. Hb(aq) + 02(aq) = HbO;(aq) K. = 1.8 Hb(aq) + CO(aq) = HBCO(aq) K = 306 Suppose that an air mixture becomes polluted with carbon monoxide at a level of 0.20%. Assuming the air contains 20.0% oxygen and that the oxygen and carbon monoxide ratios that dissolve in the blood are identical to the ratios in the air, what is the ratio of HBCO to HbO; in the bloodstream? Based on your calculations, just how toxic is carbon monoxide?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.40PAE: Because carbonic acid undergoes a second ionization, the student in Exercise 12.39 is concerned that...
Related questions
Question
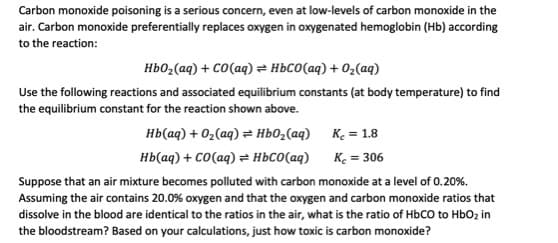
Transcribed Image Text:Carbon monoxide poisoning is a serious concern, even at low-levels of carbon monoxide in the
air. Carbon monoxide preferentially replaces oxygen in oxygenated hemoglobin (Hb) according
to the reaction:
Hb0,(aq) + CO(aq) = HBCO(aq) + 02(aq)
Use the following reactions and associated equilibrium constants (at body temperature) to find
the equilibrium constant for the reaction shown above.
нь(ад) + 0,(аg) ӕ ньо,(аg) К. 3 1.8
нЬ ад) + со(аq) ӕ ньсо(ад)
Ke = 306
Suppose that an air mixture becomes polluted with carbon monoxide at a level of 0.20%.
Assuming the air contains 20.0% oxygen and that the oxygen and carbon monoxide ratios that
dissolve in the blood are identical to the ratios in the air, what is the ratio of HbCO to HbOz in
the bloodstream? Based on your calculations, just how toxic is carbon monoxide?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning