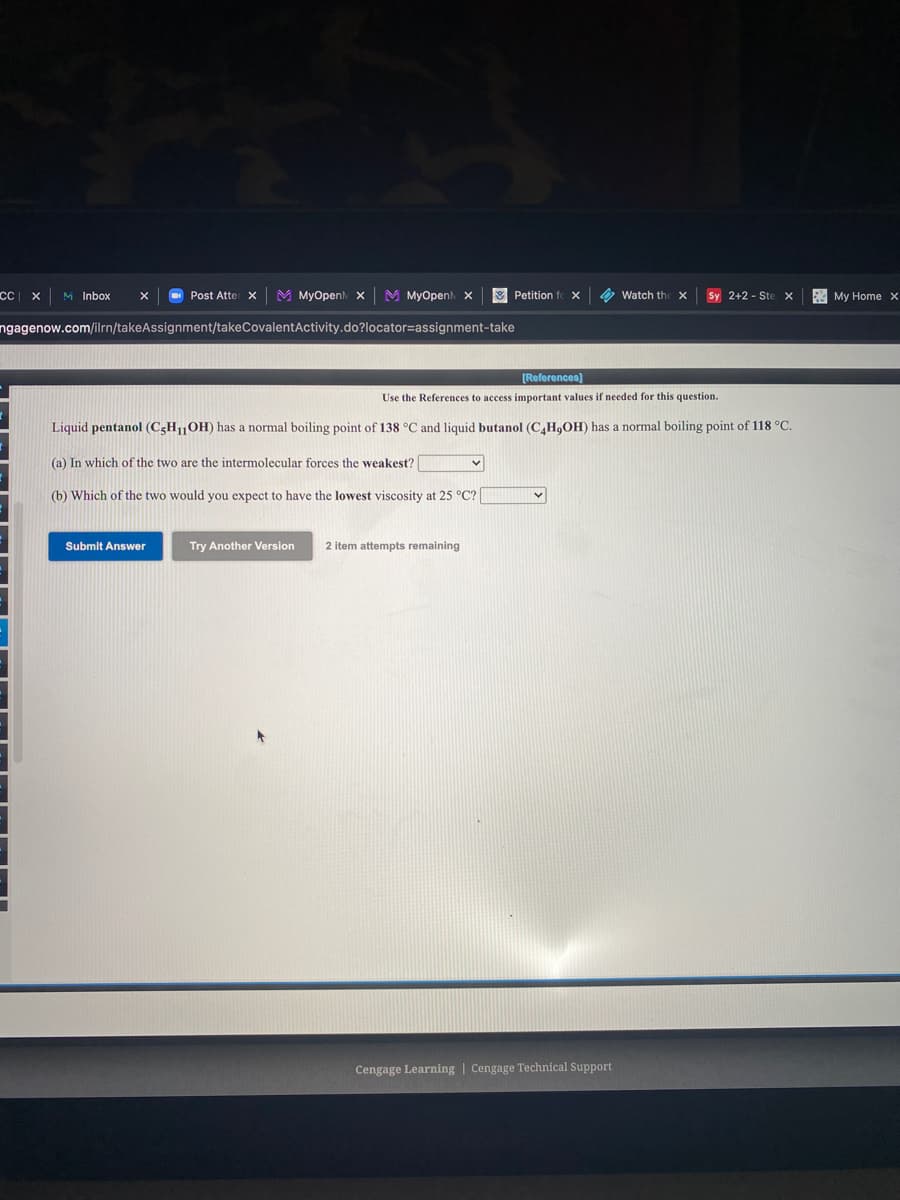
CC x M Inbox O Post Atte x M MyOpen x M MyOpenh x Petition f X Watch the X Sy 2+2 - Ste x My Home x ngagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take (References) Use the References to access important values if needed for this question. Liquid pentanol (CgH,OH) has a normal boiling point of 138 °C and liquid butanol (C,H,OH) has a normal boiling point of 118 °C. (a) In which of the two are the intermolecular forces the weakest? (b) Which of the two would you expect to have the lowest viscosity at 25 °C? ( Submit Answer Try Another Version 2 item attempts remaining
CC x M Inbox O Post Atte x M MyOpen x M MyOpenh x Petition f X Watch the X Sy 2+2 - Ste x My Home x ngagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take (References) Use the References to access important values if needed for this question. Liquid pentanol (CgH,OH) has a normal boiling point of 138 °C and liquid butanol (C,H,OH) has a normal boiling point of 118 °C. (a) In which of the two are the intermolecular forces the weakest? (b) Which of the two would you expect to have the lowest viscosity at 25 °C? ( Submit Answer Try Another Version 2 item attempts remaining
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 92QRT
Related questions
Question
Transcribed Image Text:CC
M Inbox
O Post Atter X
M MyOpen x
M MyOpen x
E Petition f
o Watch the X
Sy 2+2 - Ste x
My Home x
ngagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take
[References)
Use the References to access important values if needed for this question.
Liquid pentanol (C5HOH) has a normal boiling point of 138 °C and liquid butanol (C,H,OH) has a normal boiling point of 118 °C.
(a) In which of the two are
intermolecular
eakest?
(b) Which of the two would you expect to have the lowest viscosity at 25 °C?
Submit Answer
Try Another Version
2 item attempts remaining
Cengage Learning | Cengage Technical Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning