The boiling point of 500.0L of water was determined to be 100.0°C. Ana has decided to increase the boiling point to 101.0°C by adding ammonium sulfate. How many grams of ammonium sulfate is required to achieve this? (Kb H20 = 0.512°C/m; MW ammonium sulfate =132.14g/mol) A. 119.12 g В. 129.04 g С. 129.12 g D. 119.04 g E. None of the above. 5atm?
The boiling point of 500.0L of water was determined to be 100.0°C. Ana has decided to increase the boiling point to 101.0°C by adding ammonium sulfate. How many grams of ammonium sulfate is required to achieve this? (Kb H20 = 0.512°C/m; MW ammonium sulfate =132.14g/mol) A. 119.12 g В. 129.04 g С. 129.12 g D. 119.04 g E. None of the above. 5atm?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 64QAP: Carbon tetrachloride (CCl4) boils at 76.8C and has a density of 1.59 g/mL. (a) A solution prepared...
Related questions
Question
Transcribed Image Text:en Page 2 of 2 Previous
Next
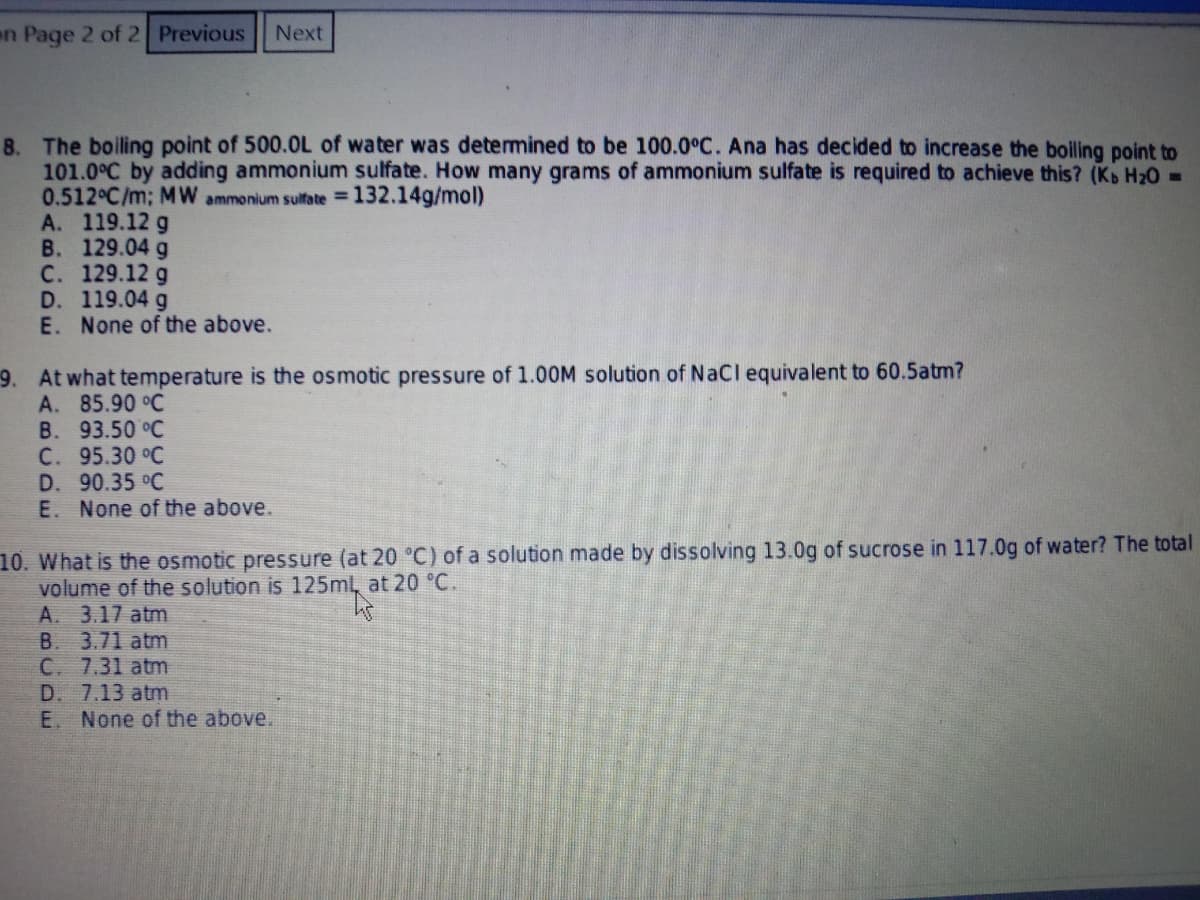
8. The boiling point of 500.OL of water was determined to be 100.0°C. Ana has decided to increase the boiling point to
101.0°C by adding ammonium sulfate. How many grams of ammonium sulfate is required to achieve this? (Kb H20 -
0.512°C/m; MW ammonium sulfate 132.14g/mol)
A. 119.12 g
В. 129.04 g
С. 129.12 g
D. 119.04 g
E. None of the above.
9. At what temperature is the osmotic pressure of 1.00M solution of NaCl equivalent to 60.5atm?
A. 85.90 °C
B. 93.50 °C
С. 95.30 °C
D. 90.35 °C
E. None of the above.
10. What is the osmotic pressure (at 20 °C) of a solution made by dissolving 13.0g of sucrose in 117.0g of water? The total
volume of the solution is 125ml, at 20 °C.
A. 3.17 atm
B. 3.71 atm
C. 7.31 atm
D. 7.13 atm
E. None of the above.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning