CD et bookmarks schoology.Isr7.org/common-assessment-delivery/start/6727737558?action=onresume&submissionid=1150090585 Heat of Solution Lab Assessment the mcAT. School Stuff A certain amount of CaCl₂ was added to 49.05 g of water and the temperature rose from 22.0°C to 41.5°C. Calculate "q" for this reaction assuming the specific heat capacity of water is 4.184 J/g°C. The heat of the reaction is the opposite sign for the heat (q) of the water hence the negative sign in front of Greaction=-mc AT Creaction=- greaction= J g)( J/g°C)( °C) DELL 1 2 3 4
CD et bookmarks schoology.Isr7.org/common-assessment-delivery/start/6727737558?action=onresume&submissionid=1150090585 Heat of Solution Lab Assessment the mcAT. School Stuff A certain amount of CaCl₂ was added to 49.05 g of water and the temperature rose from 22.0°C to 41.5°C. Calculate "q" for this reaction assuming the specific heat capacity of water is 4.184 J/g°C. The heat of the reaction is the opposite sign for the heat (q) of the water hence the negative sign in front of Greaction=-mc AT Creaction=- greaction= J g)( J/g°C)( °C) DELL 1 2 3 4
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section16.5: Entropy And The Second Law Of Thermodynamics
Problem 16.6CE
Related questions
Question
please don't reject my question ?
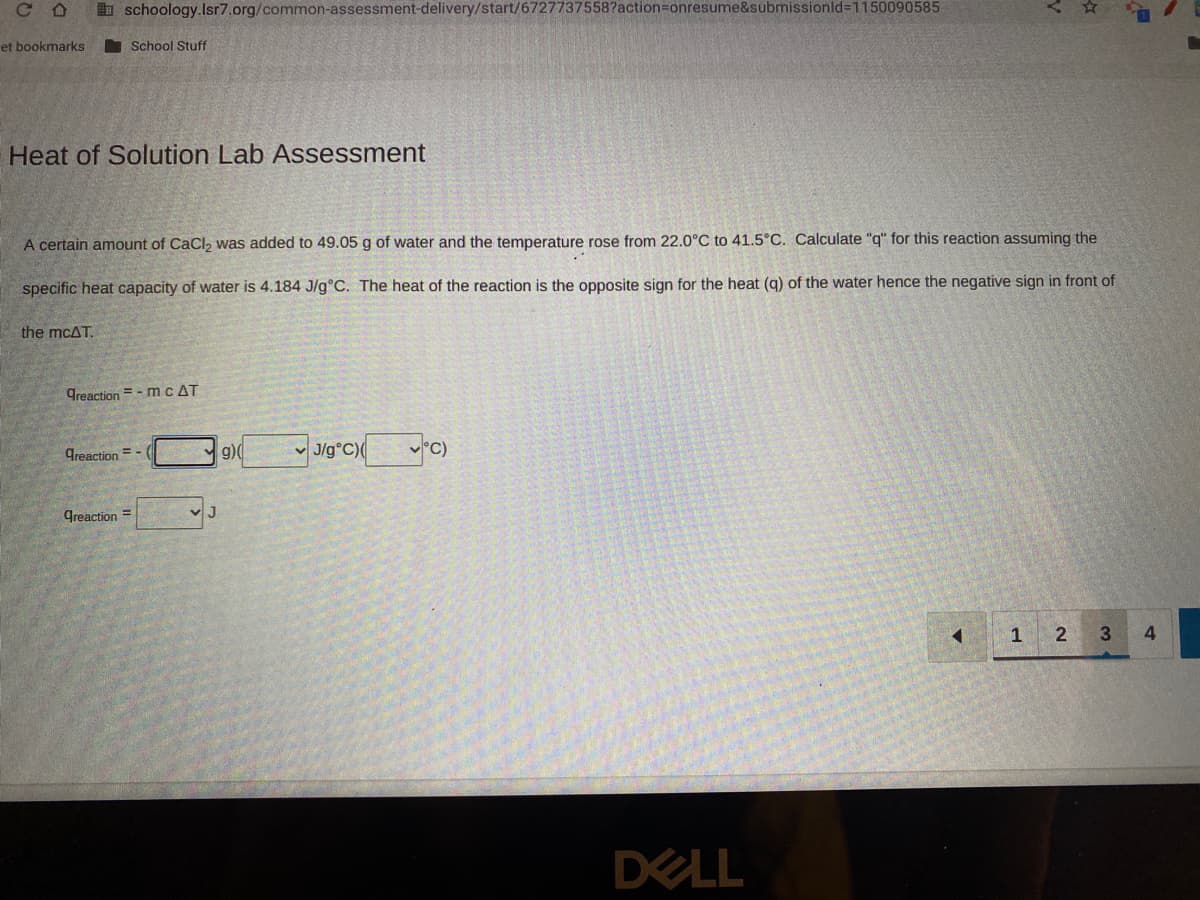
Transcribed Image Text:CD
et bookmarks
schoology.Isr7.org/common-assessment-delivery/start/6727737558?action=onresume&submissionid=1150090585
Heat of Solution Lab Assessment
the mcAT.
School Stuff
A certain amount of CaCl₂ was added to 49.05 g of water and the temperature rose from 22.0°C to 41.5°C. Calculate "q" for this reaction assuming the
specific heat capacity of water is 4.184 J/g°C. The heat of the reaction is the opposite sign for the heat (q) of the water hence the negative sign in front of
Greaction=-mc AT
Creaction=-(
greaction=
J
g)(
J/g°C)( °C)
DELL
1 2
3
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning