Certain kinds of zeolite have the general formula M2O·Al¿O3'ySiO2'wH2O, where M is an alkali metal such as sodium or potassium, y is 2 or more, and w is any inte- ger. Compute the mass percentage of aluminum in a zeo- lite that has M = K, y = 4, and w = 6.
Certain kinds of zeolite have the general formula M2O·Al¿O3'ySiO2'wH2O, where M is an alkali metal such as sodium or potassium, y is 2 or more, and w is any inte- ger. Compute the mass percentage of aluminum in a zeo- lite that has M = K, y = 4, and w = 6.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 106E: The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a...
Related questions
Question
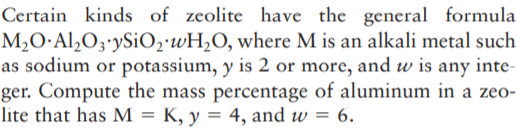
Transcribed Image Text:Certain kinds of zeolite have the general formula
M2O·Al¿O3'ySiO2'wH2O, where M is an alkali metal such
as sodium or potassium, y is 2 or more, and w is any inte-
ger. Compute the mass percentage of aluminum in a zeo-
lite that has M = K, y = 4, and w = 6.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning