C,H16 () →7 CO2 (g + 8 H2O (g) + 11 O2 (g) + 8 H2O (e) If 0.0035 mol of O2 reacts, how many mol of CO2 will be formed? Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for thi problem. Abbreviate units as follows: grams = g, moles = mol. Use the three blanks to enter the number, unit, and substance (in this order) that appears in the numerator of the stoichiometry conversion factor.
C,H16 () →7 CO2 (g + 8 H2O (g) + 11 O2 (g) + 8 H2O (e) If 0.0035 mol of O2 reacts, how many mol of CO2 will be formed? Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for thi problem. Abbreviate units as follows: grams = g, moles = mol. Use the three blanks to enter the number, unit, and substance (in this order) that appears in the numerator of the stoichiometry conversion factor.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.161QP
Related questions
Question
I don’t know how to solve this problem please help
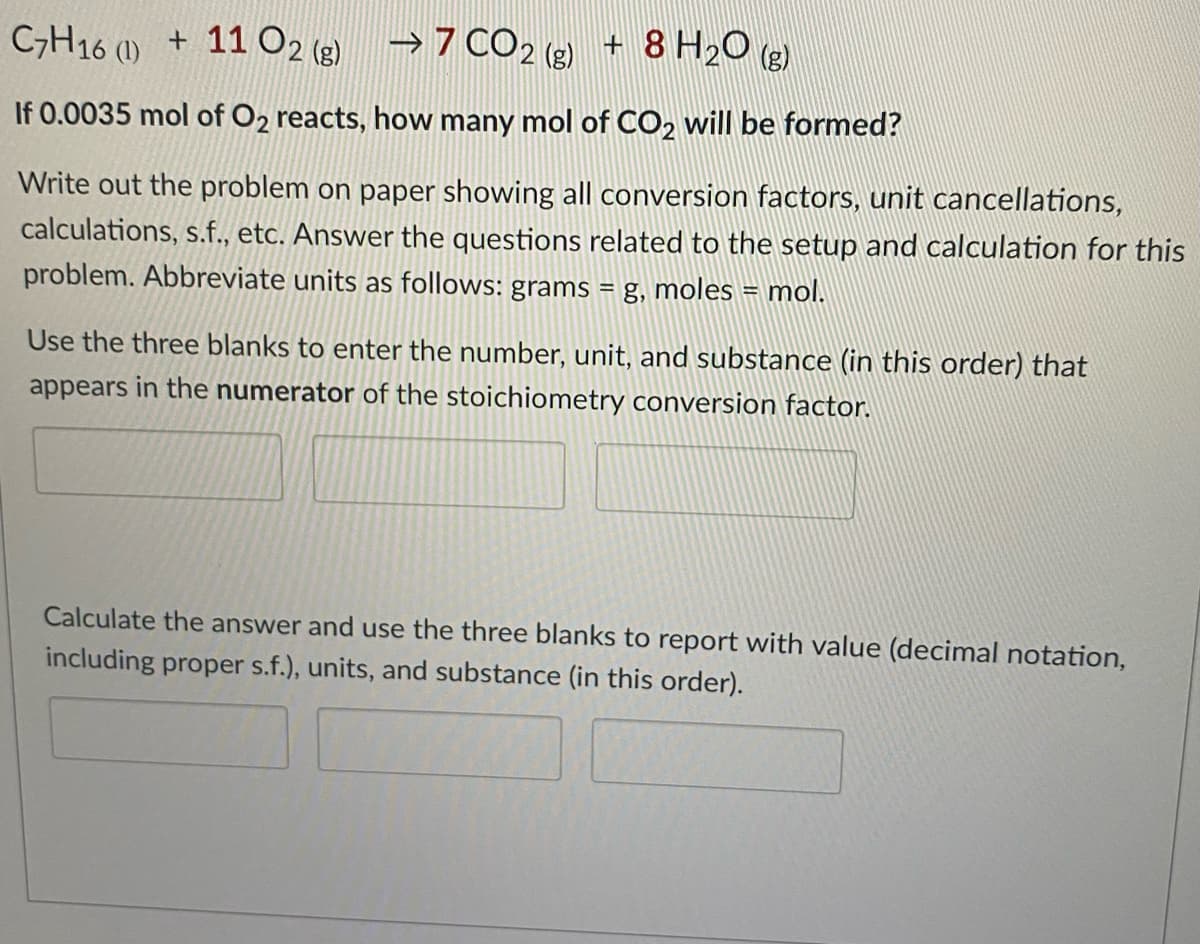
Transcribed Image Text:CH16 () + 11 O2 (g) →7 CO2 (g + 8 H2O ()
+ 8 H2O (e)
If 0.0035 mol of O2 reacts, how many mol of CO2 will be formed?
Write out the problem on paper showing all conversion factors, unit cancellations,
calculations, s.f., etc. Answer the questions related to the setup and calculation for this
problem. Abbreviate units as follows: grams = g, moles = mol.
Use the three blanks to enter the number, unit, and substance (in this order) that
appears in the numerator of the stoichiometry conversion factor.
Calculate the answer and use the three blanks to report with value (decimal notation,
including proper s.f.), units, and substance (in this order).
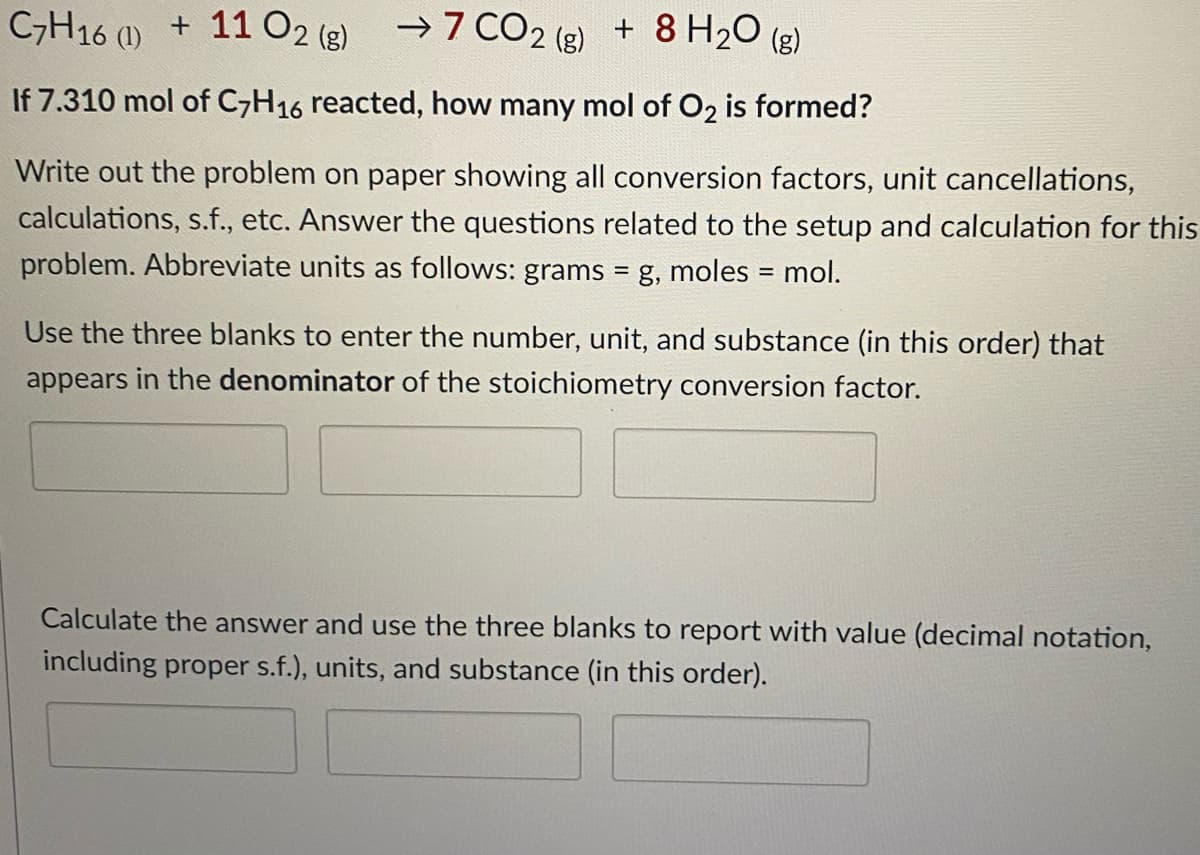
Transcribed Image Text:C,H16 (1) + 1102 (g)
→7 CO2 (g)
+ 8 H20 (g)
If 7.310 mol of C,H16 reacted, how many mol of O2 is formed?
Write out the problem on paper showing all conversion factors, unit cancellations,
calculations, s.f., etc. Answer the questions related to the setup and calculation for this
problem. Abbreviate units as follows: grams = g, moles = mol.
Use the three blanks to enter the number, unit, and substance (in this order) that
appears in the denominator of the stoichiometry conversion factor.
Calculate the answer and use the three blanks to report with value (decimal notation,
including proper s.f.), units, and substance (in this order).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning