CH3CH2CH2 C=C CH3 H CH;CH2CH2 CH3 H2C=CHCH2CH2CH2CH3 C=C H H A B Which of the alkenes above is the least stable (highest in energy)? | Which is the most stable (lowest in energy)? |
CH3CH2CH2 C=C CH3 H CH;CH2CH2 CH3 H2C=CHCH2CH2CH2CH3 C=C H H A B Which of the alkenes above is the least stable (highest in energy)? | Which is the most stable (lowest in energy)? |
Chapter3: Organic Compounds: Alkanes And Their Stereochemistry
Section3.SE: Something Extra
Problem 46AP: Draw the most stable conformation of pentane, using wedges and dashes to represent bonds coming out...
Related questions
Question
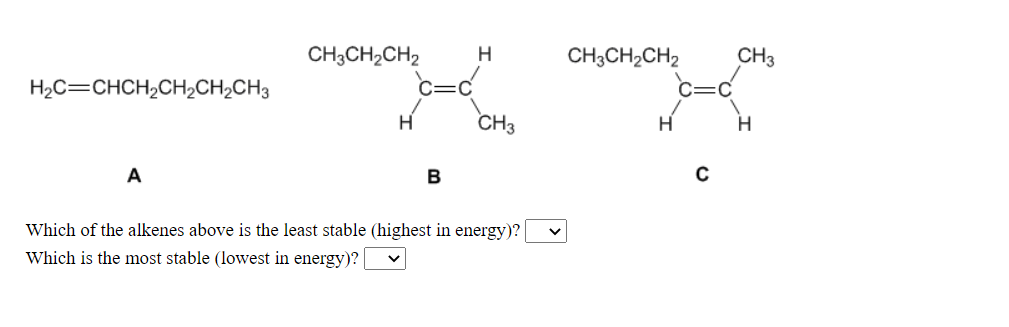
Transcribed Image Text:CH3CH2CH2
H
CH3CH2CH2
CH3
H2C=CHCH,CH2CH2CH3
CH3
B
Which of the alkenes above is the least stable (highest in energy)?
Which is the most stable (lowest in energy)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning