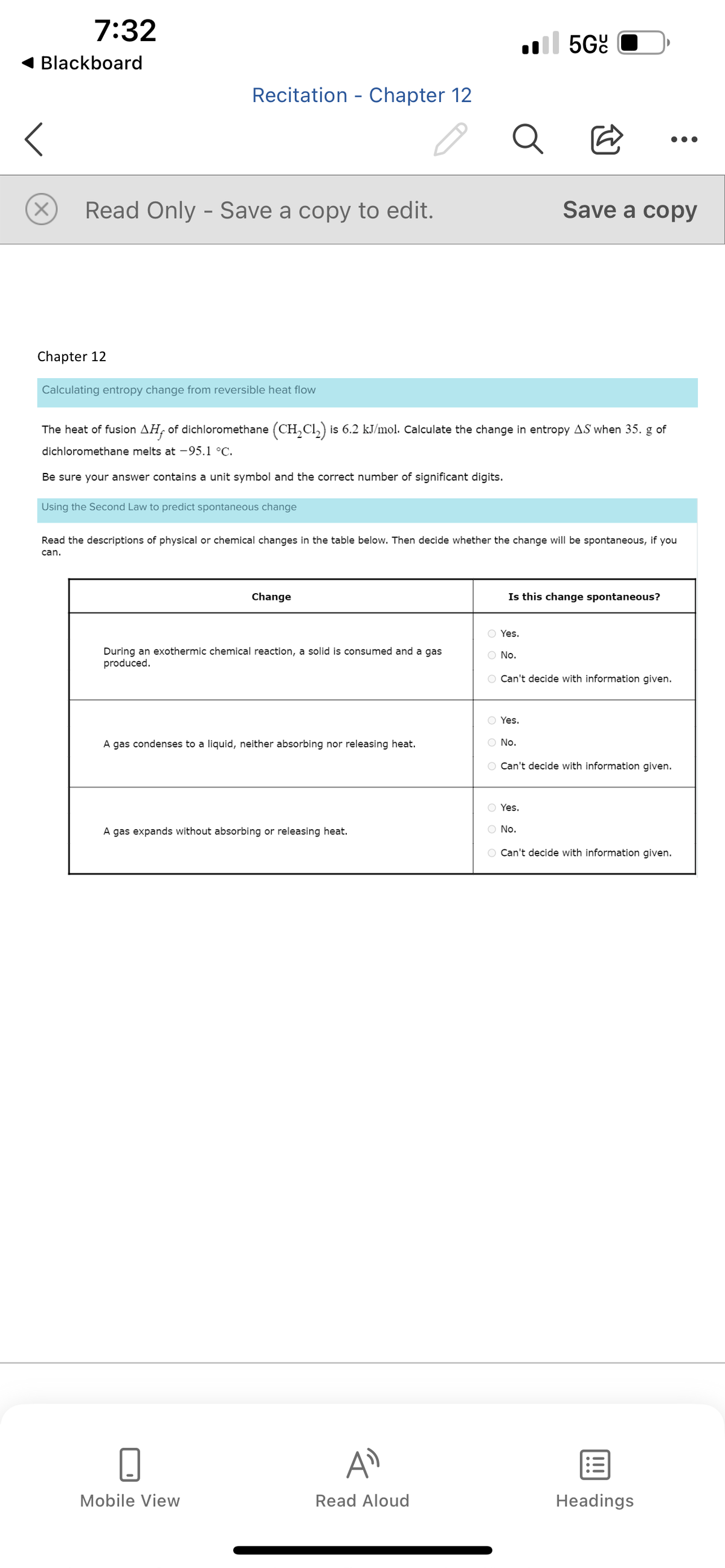
Chapter 12 Calculating entropy change from reversible heat flow The heat of fusion AH, of dichloromethane (CH₂Cl₂) is 6.2 kJ/mol. Calculate the change in entropy AS when 35. g of dichloromethane melts at -95.1 °C. Be sure your answer contains a unit symbol and the correct number of significant digits.
Chapter 12 Calculating entropy change from reversible heat flow The heat of fusion AH, of dichloromethane (CH₂Cl₂) is 6.2 kJ/mol. Calculate the change in entropy AS when 35. g of dichloromethane melts at -95.1 °C. Be sure your answer contains a unit symbol and the correct number of significant digits.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.29PAE
Related questions
Question
Transcribed Image Text:7:32
Blackboard
Chapter 12
Recitation Chapter 12
X Read Only - Save a copy to edit.
Calculating entropy change from reversible heat flow
Using the Second Law to predict spontaneous change
-
Change
The heat of fusion AH, of dichloromethane (CH₂Cl₂) is 6.2 kJ/mol. Calculate the change in entropy AS when 35. g of
dichloromethane melts at -95.1 °C.
Be sure your answer contains a unit symbol and the correct number of significant digits.
During an exothermic chemical reaction, a solid is consumed and a gas
produced.
Mobile View
A gas condenses to a liquid, neither absorbing nor releasing heat.
A gas expands without absorbing or releasing heat.
Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you
can.
Q
AD
Read Aloud
.5Gº
O Yes.
O No.
@
Save a copy
Is this change spontaneous?
O Yes.
O No.
O Can't decide with information given.
O Can't decide with information given.
O Yes.
O No.
O Can't decide with information given.
Headings
Transcribed Image Text:7:32
Blackboard
×
Read Only - Save a copy to edit.
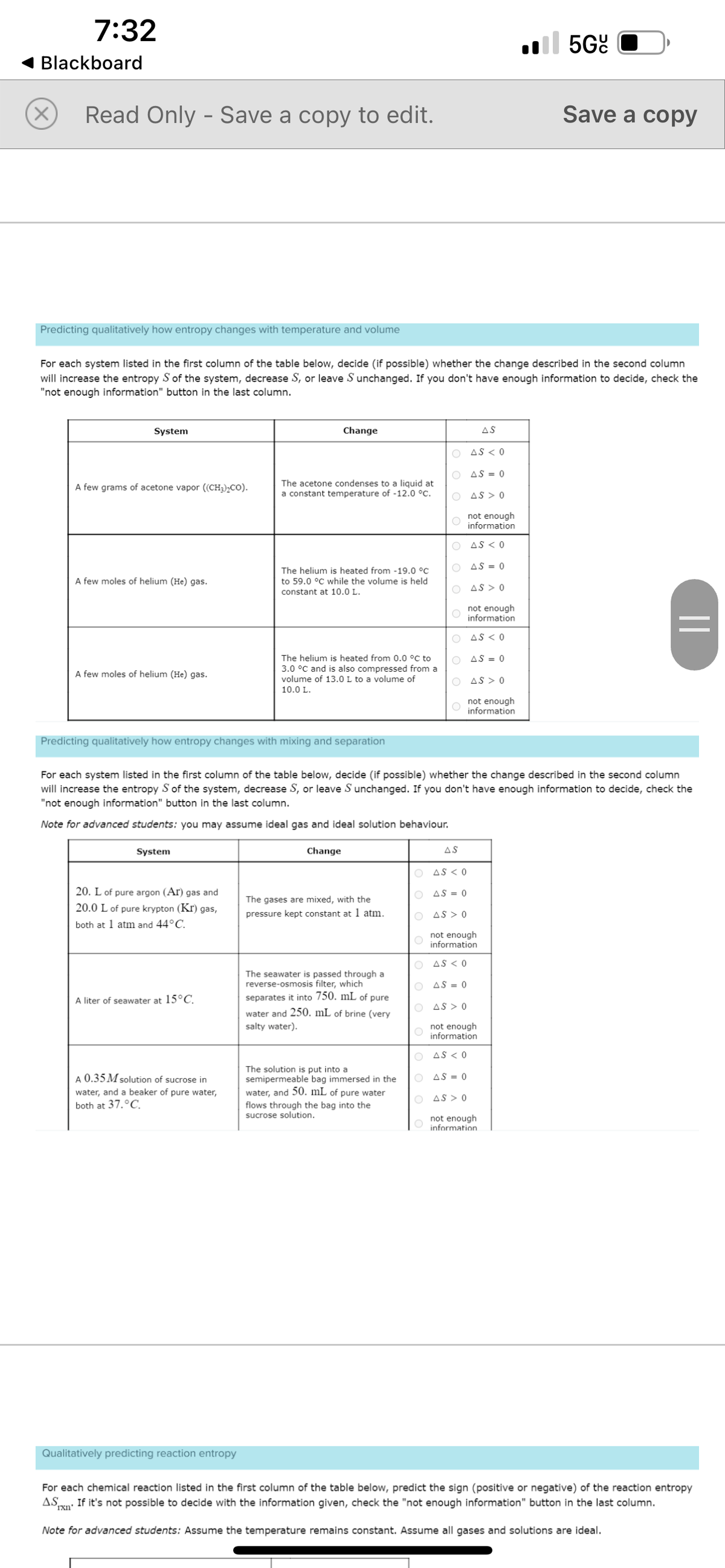
Predicting qualitatively how entropy changes with temperature and volume
System
For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column
will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the
"not enough information" button in the last column.
A few grams of acetone vapor ((CH3)₂CO).
A few moles of helium (He) gas.
A few moles of helium (He) gas.
System
20. L of pure argon (Ar) gas and
20.0 L of pure krypton (Kr) gas,
both at 1 atm and 44°C.
Predicting qualitatively how entropy changes with mixing and separation
A liter of seawater at 15°C.
Change
A 0.35 M solution of sucrose in
water, and a beaker of pure water,
both at 37.°C.
The acetone condenses to a liquid at
a constant temperature of -12.0 °C.
Qualitatively predicting reaction entropy
The helium is heated from -19.0 °C
to 59.0 °C while the volume is held
constant at 10.0 L.
The helium is heated from 0.0 °C to
3.0 °C and is also compressed from a
volume of 13.0 L to a volume of
10.0 L.
The gases are mixed, with the
pressure kept constant at 1 atm.
The seawater is passed through a
reverse-osmosis filter, which
separates it into 750. mL of pure
water and 250. mL of brine (very
salty water).
The solution is put into a
semipermeable bag immersed in the
water, and 50. mL of pure water
flows through the bag into the
sucrose solution.
AS
OAS < 0
O
AS = 0
O
AS > 0
not enough
information
O
For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column
will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the
"not enough information" button in the last column.
Note for advanced students: you may assume ideal gas and ideal solution behaviour.
Change
O
OAS < 0
OAS=0
O AS > 0
O
OAS < 0
O AS = 0
OAS 0
AS
OAS < 0
OAS = 0
O AS> 0
not enough
information
ΤΟ ΔS < 0
OAS=0
O AS >0
not enough
information
not enough
information
OAS <0
OAS = 0
O AS> 0
not enough
information
.5Gc
Save a copy
not enough
information
||
For each chemical reaction listed in the first column of the table below, predict the sign (positive or negative) of the reaction entropy
AS If it's not possible to decide with the information given, check the "not enough information" button in the last column.
rxn'
Note for advanced students: Assume the temperature remains constant. Assume all gases and solutions are ideal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning