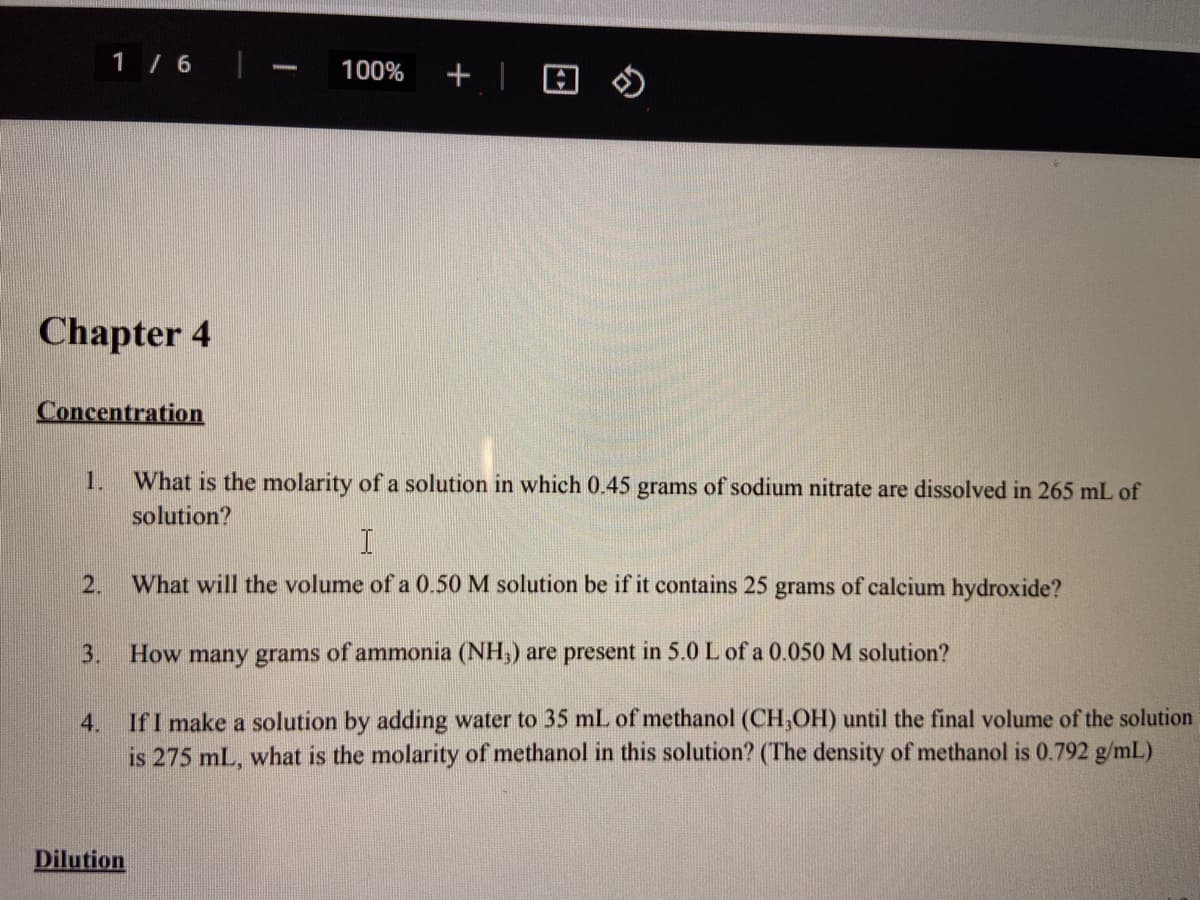
Chapter 4 Concentration 1. What is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 mL of solution? 2. What will the volume of a 0.50 M solution be if it contains 25 grams of calcium hydroxide? 3. How many grams of ammonia (NH,) are present in 5.0 L of a 0.050 M solution? If I make a solution by adding water to 35 mL of methanol (CH,OH) until the final volume of the solution is 275 mL, what is the molarity of methanol in this solution? (The density of methanol is 0.792 g/mL) 4. Dilution
Chapter 4 Concentration 1. What is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 mL of solution? 2. What will the volume of a 0.50 M solution be if it contains 25 grams of calcium hydroxide? 3. How many grams of ammonia (NH,) are present in 5.0 L of a 0.050 M solution? If I make a solution by adding water to 35 mL of methanol (CH,OH) until the final volume of the solution is 275 mL, what is the molarity of methanol in this solution? (The density of methanol is 0.792 g/mL) 4. Dilution
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 101AP
Related questions
Question
100%
Questions 1 &4 from image
Transcribed Image Text:1 / 6
100%
の
Chapter 4
Concentration
1.
What is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 mL of
solution?
2.
What will the volume of a 0.50 M solution be if it contains 25 grams of calcium hydroxide?
3.
How many grams of ammonia (NH,) are present in 5.0 L of a 0.050 M solution?
If I make a solution by adding water to 35 mL of methanol (CH,OH) until the final volume of the solution
is 275 mL, what is the molarity of methanol in this solution? (The density of methanol is 0.792 g/mL)
4.
Dilution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
