Chemical energy is released or absorbed from reactions in various forms. The most easily measurable form of energy comes in the form of heat, or enthalpy. The enthalpy of a reaction can be calculated from the heats of formation of the substances involved in the reaction: A,H° = E npAf H° (products) – E nAf H° (reactants) where n represents the stoichiometric coefficients. Пр.
Chemical energy is released or absorbed from reactions in various forms. The most easily measurable form of energy comes in the form of heat, or enthalpy. The enthalpy of a reaction can be calculated from the heats of formation of the substances involved in the reaction: A,H° = E npAf H° (products) – E nAf H° (reactants) where n represents the stoichiometric coefficients. Пр.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 19Q: The equation for the fermentation of glucose to alcohol and carbon dioxide is: C6H12O6(aq) ...
Related questions
Question
please answer the question with clarity

Transcribed Image Text:Chemical energy is released or absorbed from reactions in
various forms. The most easily measurable form of energy
comes in the form of heat, or enthalpy. The enthalpy of a
reaction can be calculated from the heats of formation of the
substances involved in the reaction:
Δ. ΗΣ Τ, Δ Η' (products)-Σ η, Δ Η' (reactants)
where n represents the stoichiometric coefficients.
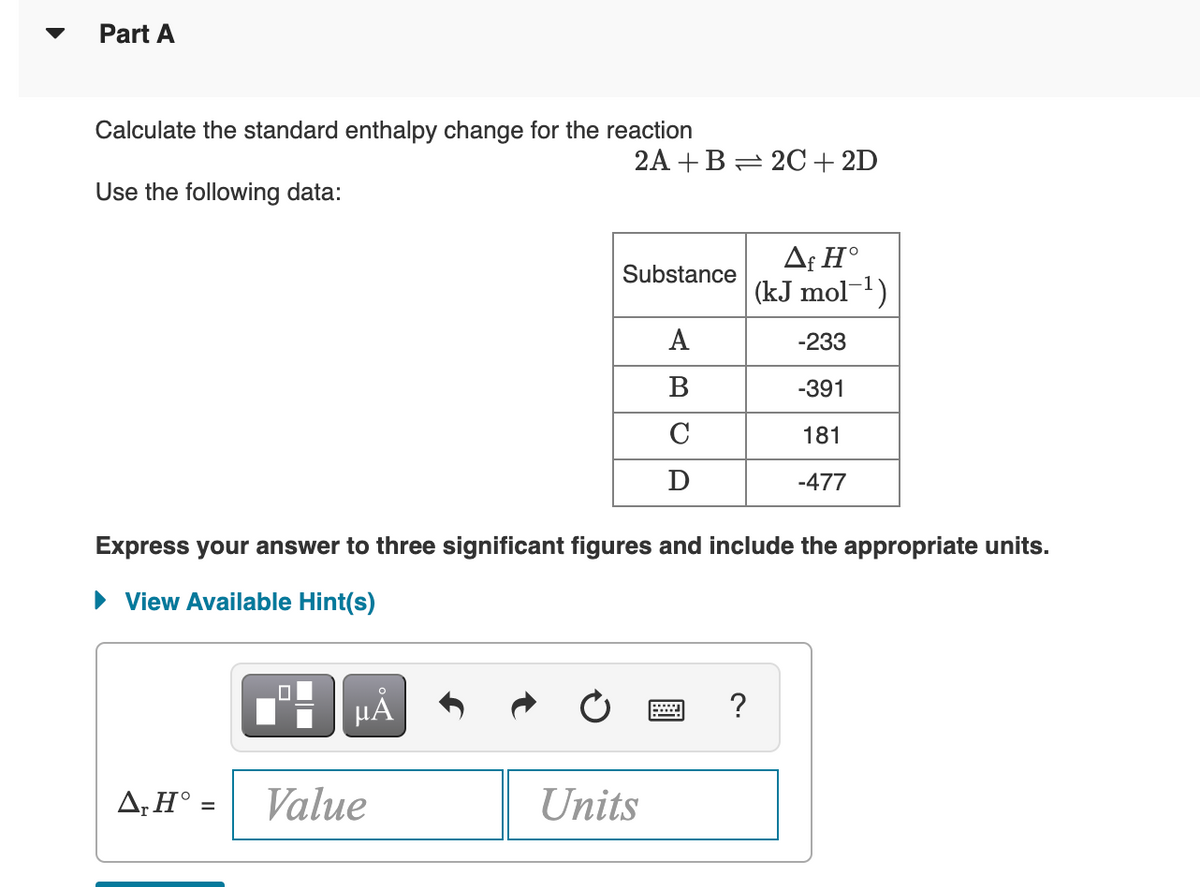
Transcribed Image Text:Part A
Calculate the standard enthalpy change for the reaction
2A +B= 2C+2D
Use the following data:
Af H°
(kJ mol-1)
Substance
A
-233
B
-391
C
181
D
-477
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
HA
A,H° =
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning