Chemical reaction often follows the model: dc = -kc" dt where c = concentration, t = time, k = reaction rate and n = reaction order. Using the fundamental of logarithmic, the above reaction can be written as: log (-) dc log k +n logc %3D dt Use this approach along with the following data to find the value of k and n at time (t) 20 and 45 respectively. 10 20 40 3.52 2.48 1.23 t 15 30 45 60 75 3.17 1.75 1.02 0.61 0.43
Chemical reaction often follows the model: dc = -kc" dt where c = concentration, t = time, k = reaction rate and n = reaction order. Using the fundamental of logarithmic, the above reaction can be written as: log (-) dc log k +n logc %3D dt Use this approach along with the following data to find the value of k and n at time (t) 20 and 45 respectively. 10 20 40 3.52 2.48 1.23 t 15 30 45 60 75 3.17 1.75 1.02 0.61 0.43
Chapter6: Exponential And Logarithmic Functions
Section6.6: Exponential And Logarithmic Equations
Problem 79SE: Recall the formula for continually compoundinginterest, y=Aekt. Use the definition of a...
Related questions
Question
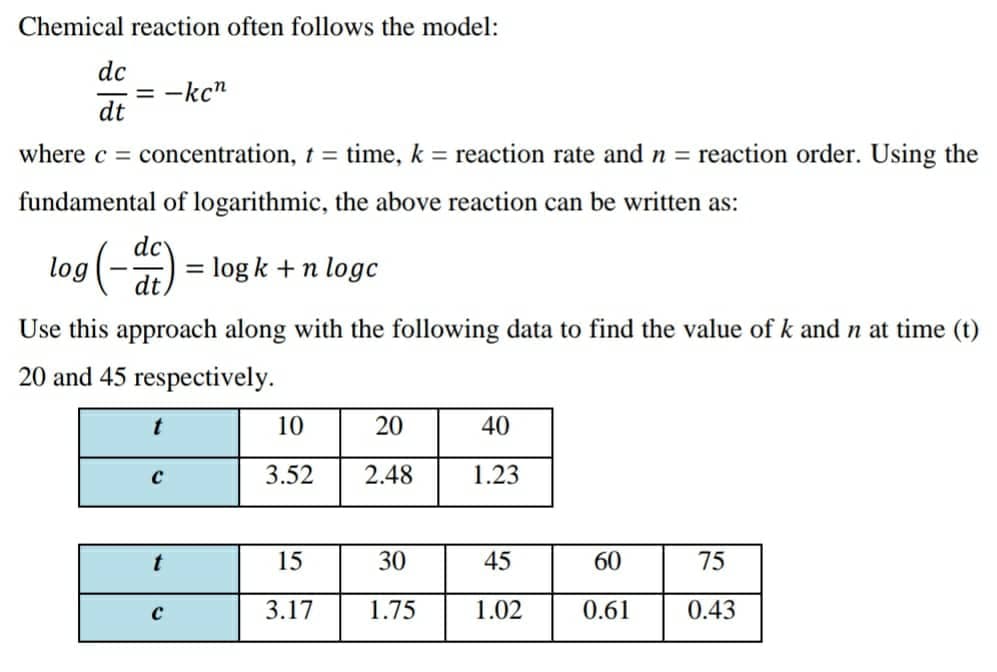
Transcribed Image Text:Chemical reaction often follows the model:
dc
= -kc"
dt
where c =
concentration, t = time, k = reaction rate and n = reaction order. Using the
fundamental of logarithmic, the above reaction can be written as:
dc
log (-) = log k + n loge
%3D
dt
Use this approach along with the following data to find the value of k andn at time (t)
20 and 45 respectively.
t
10
20
40
3.52
2.48
1.23
t
15
30
45
60
75
3.17
1.75
1.02
0.61
0.43
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, statistics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

College Algebra (MindTap Course List)
Algebra
ISBN:
9781305652231
Author:
R. David Gustafson, Jeff Hughes
Publisher:
Cengage Learning


Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

College Algebra (MindTap Course List)
Algebra
ISBN:
9781305652231
Author:
R. David Gustafson, Jeff Hughes
Publisher:
Cengage Learning

Algebra and Trigonometry (MindTap Course List)
Algebra
ISBN:
9781305071742
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning
