Chemicals Amount 1,4-dimethoxyBenzene 0.327 g (2.37 *10^-3 mol) t-butyl alcohol (density=0.781 g/mL) 0.5 mL (5.27*10^-3mol) Conc. Sulfuric Acid 1.0 mL 1,4-di-t-butyl-2,5-dimethoxybenzene 0.247 g (9.86*10^-4mol) Observed melting point 103-104 degrees C Percentage yield- Mass Melting point|
Chemicals Amount 1,4-dimethoxyBenzene 0.327 g (2.37 *10^-3 mol) t-butyl alcohol (density=0.781 g/mL) 0.5 mL (5.27*10^-3mol) Conc. Sulfuric Acid 1.0 mL 1,4-di-t-butyl-2,5-dimethoxybenzene 0.247 g (9.86*10^-4mol) Observed melting point 103-104 degrees C Percentage yield- Mass Melting point|
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 9ALQ: In lab you report a measured volume of 128.7 mL of water. Using significant figures as a measure of...
Related questions
Question
help with the percentage yield and melting point
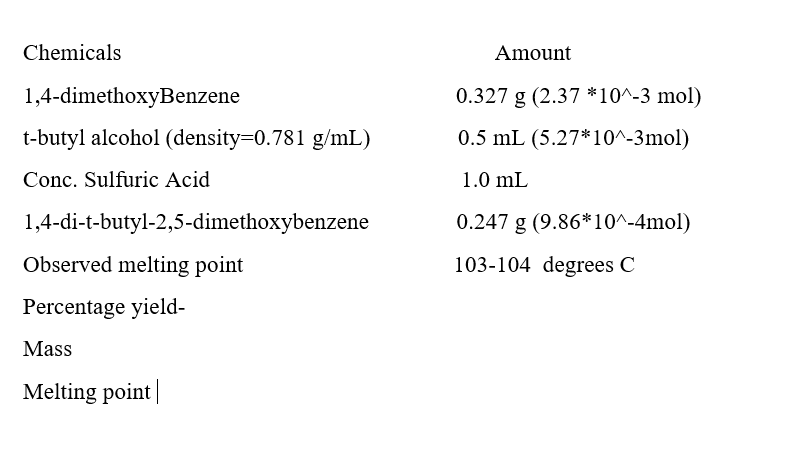
Transcribed Image Text:Chemicals
Amount
1,4-dimethoxyBenzene
0.327 g (2.37 *10^-3 mol)
t-butyl alcohol (density=D0.781 g/mL)
0.5 mL (5.27*10^-3mol)
Conc. Sulfuric Acid
1.0 mL
1,4-di-t-butyl-2,5-dimethoxybenzene
0.247 g (9.86*10^-4mol)
Observed melting point
103-104 degrees C
Percentage yield-
Mass
Melting point|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning