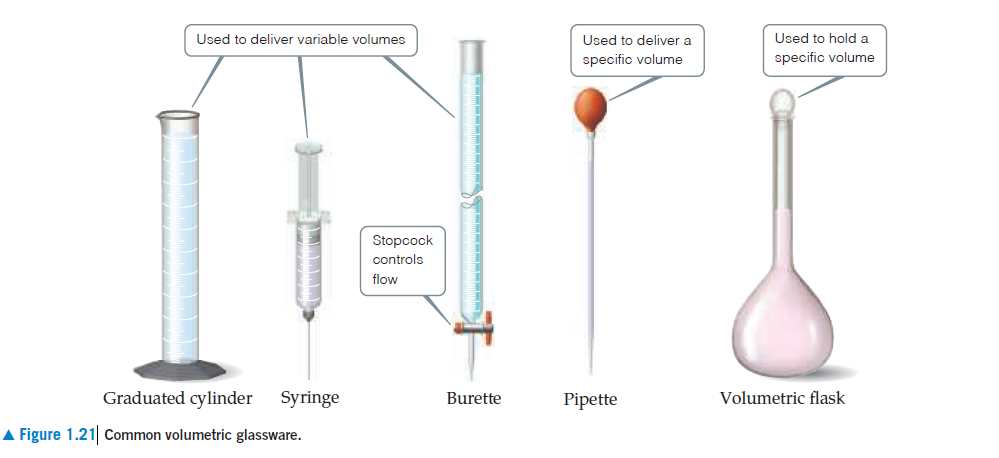
Used to deliver variable volumes Used to hold a Used to deliver a specific volume specific volume Stopcock controls flow Graduated cylinder Syringe Volumetric flask Burette Pipette A Figure 1.21 Common volumetric glassware.
(a) To identify a liquid substance, a student determined its
density. Using a graduated cylinder, she measured out a 45-mL
sample of the substance. She then measured the mass of the
sample, finding that it weighed 38.5 g. She knew that the substance
had to be either isopropyl alcohol (density 0.785 g/mL)
or toluene (density 0.866 g/mL). What are the calculated density
and the probable identity of the substance? (b) An experiment
requires 45.0 g of ethylene glycol, a liquid whose density
is 1.114 g/mL. Rather than weigh the sample on a balance, a
chemist chooses to dispense the liquid using a graduated cylinder.
What volume of the liquid should he use? (c) Is a graduated
cylinder such as that shown in Figure 1.21 likely to afford the
accuracy of measurement needed? (d) A cubic piece of metal
measures 5.00 cm on each edge. If the metal is nickel, whose
density is 8.90 g/cm3, what is the mass of the cube?
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images









