Substance a) Draw the best Lewis structure(s), resonances, and structural isomers if any b) In your structure above, indicate polar bonds with dipole arrows toward the more electronegative atom c) Include formal charges if they are not zero Name the electronic around geometry central atom(s) Give hybridization for central atom(s) Name the shape around central atom(s) Show 3-D sketch of the structure and label all bond angles How many sigma bonds? How many pi bonds? Is the substance an ionic compound, a polar molecule, a nonpolar molecule, or a polyatomic ion? Complete the following table for the indicated species: C6H6 (ring) S8 PO43 C3H8O
Substance a) Draw the best Lewis structure(s), resonances, and structural isomers if any b) In your structure above, indicate polar bonds with dipole arrows toward the more electronegative atom c) Include formal charges if they are not zero Name the electronic around geometry central atom(s) Give hybridization for central atom(s) Name the shape around central atom(s) Show 3-D sketch of the structure and label all bond angles How many sigma bonds? How many pi bonds? Is the substance an ionic compound, a polar molecule, a nonpolar molecule, or a polyatomic ion? Complete the following table for the indicated species: C6H6 (ring) S8 PO43 C3H8O
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section: Chapter Questions
Problem 71SCQ: Bromine forms a number of oxides of varying stability. (a) One oxide has 90.90% Br and 9.10% O....
Related questions
Question
Please help wiith the fourth one (C3H8O)
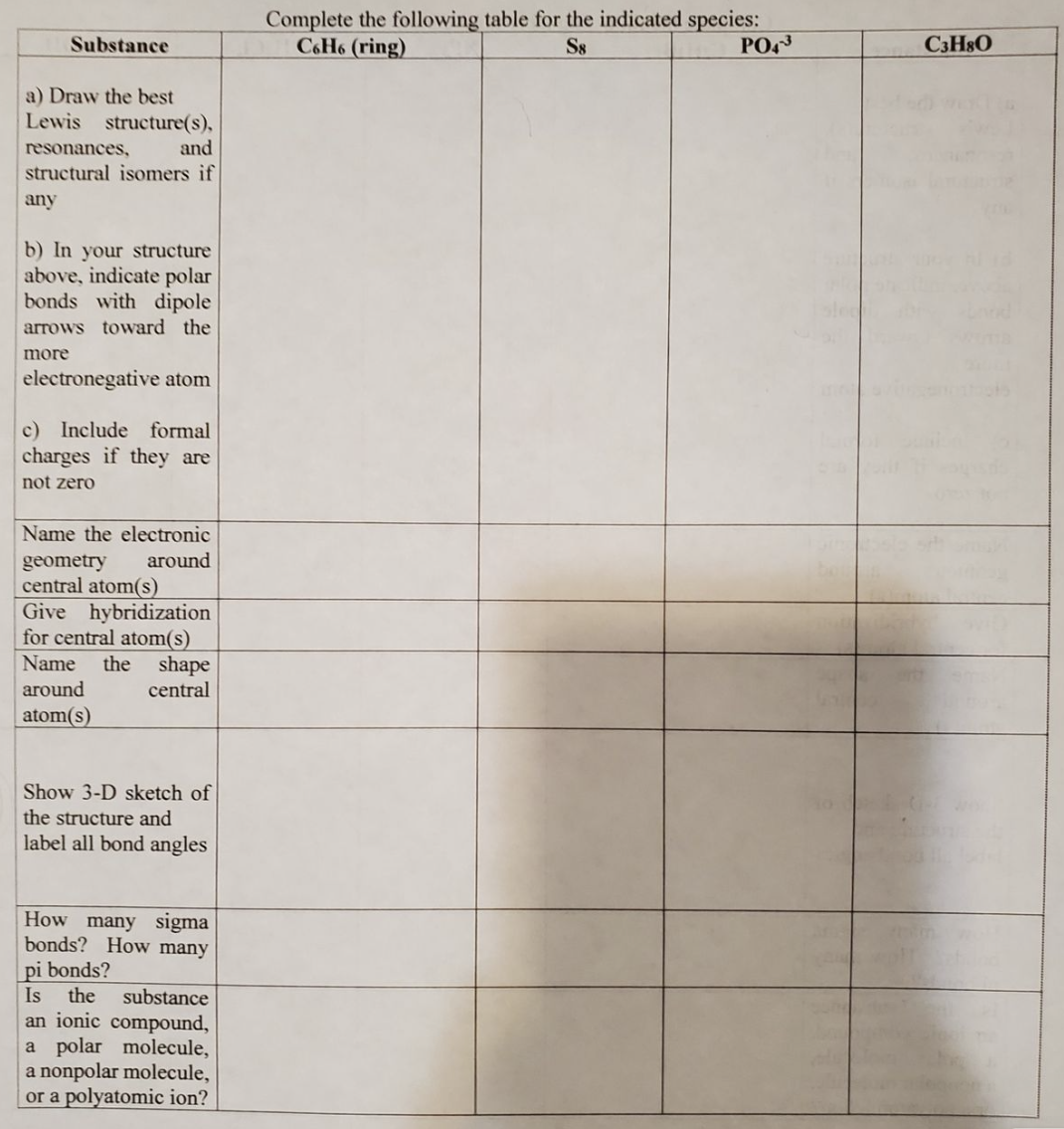
Transcribed Image Text:Substance
a) Draw the best
Lewis structure(s),
resonances,
and
structural isomers if
any
b) In your structure
above, indicate polar
bonds with dipole
arrows toward the
more
electronegative atom
c) Include formal
charges if they are
not zero
Name the electronic
around
geometry
central atom(s)
Give hybridization
for central atom(s)
Name the shape
around
central
atom(s)
Show 3-D sketch of
the structure and
label all bond angles
How many sigma
bonds? How many
pi bonds?
Is the substance
an ionic compound,
a polar molecule,
a nonpolar molecule,
or a polyatomic ion?
Complete the following table for the indicated species:
C6H6 (ring)
S8
PO43
C3H8O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax