1.1 Weigh out 1.0312 g of sodium hydroxide flakes in a clean and dry 100 mL beaker. 1.2 Add enough distilled water to the beaker to dissolve the NaOH flakes. Use a glass stirrer if necessary. (Caution: Solution is exothermic, or it gets hot as material dissolves!) 1.3 Transfer the liquid portion of the solution into a clean 250 ml volumetric flask. Add a little water to the beaker enough to dissolve any remaining solids and then add the solution to the volumetric flask. Repeat this until there are no more residues left in the beaker, but make sure that the total amount of water added to the volumetric flask does not reach the level of the calibration mark. If this happens, discard the whole solution and start over from 1.1. 1.4 Using a dropper, carefully add distilled water to the volumetric flask until the calibration mark. Carefully mix the contents of the flask by shaking or inverting the stoppered flask several times. 1.5 After mixing, make sure that the level of the solution inside the flask is exactly at the calibration mark. Add more water if it is below the flask's calibration mark. 1.6 Transfer the NaOH solution to a clean plastic container and seal it. Carefully clean the volumetric flask with soap and water, rinsing it with tap water first and final-rinsing it with distilled water. 1.7 Calculate the molarity of the NaOH solution you just prepared. (While this concentration may be the most precise value you could come up with, it is not the most accurate. This solution must undergo standardization to determine its exact molarity, something that this level of activity does not permit.) Label the solution with the calculated molarity with the date of preparation. Preparation of titrant (NaOH) Exact weight of solid NaOH grams Volume of NaOH solution prepared milliliters Molarity of NaOH prepared moles per liter
1.1 Weigh out 1.0312 g of sodium hydroxide flakes in a clean and dry 100 mL beaker. 1.2 Add enough distilled water to the beaker to dissolve the NaOH flakes. Use a glass stirrer if necessary. (Caution: Solution is exothermic, or it gets hot as material dissolves!) 1.3 Transfer the liquid portion of the solution into a clean 250 ml volumetric flask. Add a little water to the beaker enough to dissolve any remaining solids and then add the solution to the volumetric flask. Repeat this until there are no more residues left in the beaker, but make sure that the total amount of water added to the volumetric flask does not reach the level of the calibration mark. If this happens, discard the whole solution and start over from 1.1. 1.4 Using a dropper, carefully add distilled water to the volumetric flask until the calibration mark. Carefully mix the contents of the flask by shaking or inverting the stoppered flask several times. 1.5 After mixing, make sure that the level of the solution inside the flask is exactly at the calibration mark. Add more water if it is below the flask's calibration mark. 1.6 Transfer the NaOH solution to a clean plastic container and seal it. Carefully clean the volumetric flask with soap and water, rinsing it with tap water first and final-rinsing it with distilled water. 1.7 Calculate the molarity of the NaOH solution you just prepared. (While this concentration may be the most precise value you could come up with, it is not the most accurate. This solution must undergo standardization to determine its exact molarity, something that this level of activity does not permit.) Label the solution with the calculated molarity with the date of preparation. Preparation of titrant (NaOH) Exact weight of solid NaOH grams Volume of NaOH solution prepared milliliters Molarity of NaOH prepared moles per liter
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 121AP: Calcium carbonate, CaCO3, can be obtained in a very pure state. Standard solutions of calcium ion...
Related questions
Question
100%

Transcribed Image Text:1.1 Weigh out 1.0312 g of sodium hydroxide flakes in a clean and dry 100 mL beaker.
1.2 Add enough distilled water to the beaker to dissolve the NaOH flakes. Use a glass stirrer if necessary.
(Caution: Solution is exothermic, or it gets hot as material dissolves!)
1.3 Transfer the liquid portion of the solution into a clean 250 ml volumetric flask. Add a little water to
the beaker enough to dissolve any remaining solids and then add the solution to the volumetric flask.
Repeat this until there are no more residues left in the beaker, but make sure that the total amount
of water added to the volumetric flask does not reach the level of the calibration mark. If this happens,
discard the whole solution and start over from 1.1.
1.4 Using a dropper, carefully add distilled water to the volumetric flask until the calibration mark. Carefully
mix the contents of the flask by shaking or inverting the stoppered flask several times.
1.5 After mixing, make sure that the level of the solution inside the flask is exactly at the calibration mark.
Add more water if it is below the flask's calibration mark.
1.6 Transfer the NaOH solution to a clean plastic container and seal it. Carefully clean the volumetric flask
with soap and water, rinsing it with tap water first and final-rinsing it with distilled water.
1.7 Calculate the molarity of the NaOH solution you just prepared. (While this concentration may be the
most precise value you could come up with, it is not the most accurate. This solution must undergo
standardization to determine its exact molarity, something that this level of activity does not permit.)
Label the solution with the calculated molarity with the date of preparation.
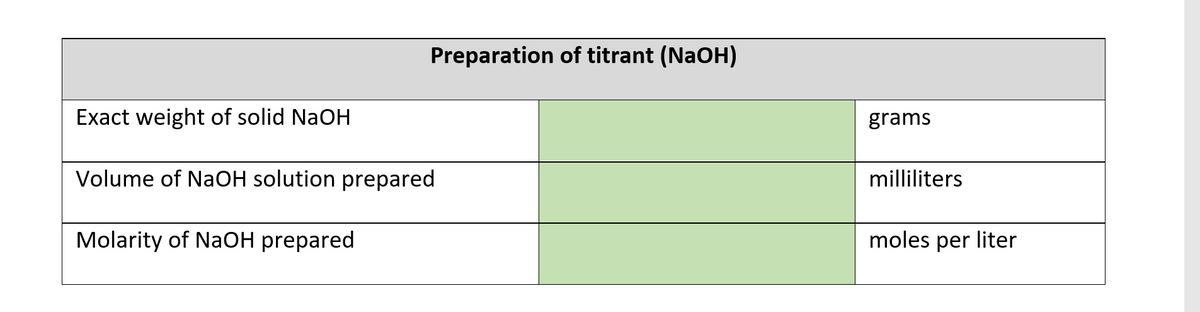
Transcribed Image Text:Preparation of titrant (NaOH)
Exact weight of solid NaOH
grams
Volume of NaOH solution prepared
milliliters
Molarity of NaOH prepared
moles per liter
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
