Lithium atom is: Cation/Anion/Neither # of protons # of electrons Charge (1-, 2-, 3-, 1+, 2+, 3+, or 0) Number of dots on the Lewis dot structure? Lose/ gain How many electrons does atom lose/gain to become more stable? electron(s)
Lithium atom is: Cation/Anion/Neither # of protons # of electrons Charge (1-, 2-, 3-, 1+, 2+, 3+, or 0) Number of dots on the Lewis dot structure? Lose/ gain How many electrons does atom lose/gain to become more stable? electron(s)
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter5: Chemical Bonding: The Covalent Bond Model
Section: Chapter Questions
Problem 5.64EP
Related questions
Question
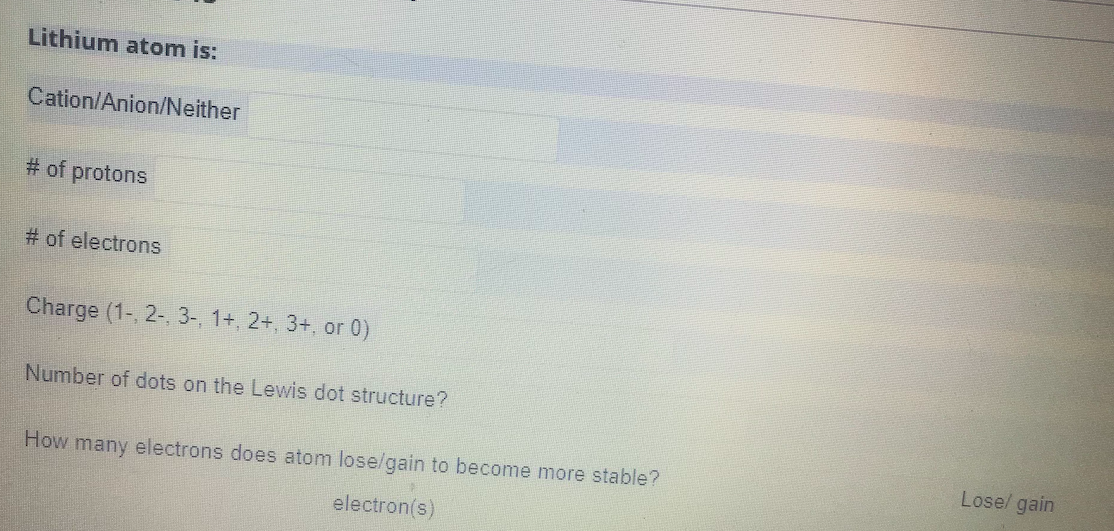
Transcribed Image Text:Lithium atom is:
Cation/Anion/Neither
# of protons
# of electrons
Charge (1-, 2-, 3-, 1+, 2+, 3+, or 0)
Number of dots on the Lewis dot structure?
Lose/ gain
How many electrons does atom lose/gain to become more stable?
electron(s)
Expert Solution
Step 1

Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning