For each part of this question, consider the following scenario: You need to make a stock solution of Mg(HC03)2 with a concentration of 5.50 M. You have a 750.0 mL volumetric flask available to make this stock solution in. You are then tasked with using this stock solution to prepare a sample for use in an experiment. The experimental sample should have a final volume of 5.00 mL and a concentration of 0.150 M Mg(HCO3)2. a) What is the molar mass of Mg(HCO3)2? b) How many moles of the solute would be needed to create the stock solution using the volumetric flask available? c) How many grams of the solute should be added to the volumetric flask to create the stock solution? d) What volume, in mL, of the stock solution should be used to prepare the experimental solution through dilution?
For each part of this question, consider the following scenario: You need to make a stock solution of Mg(HC03)2 with a concentration of 5.50 M. You have a 750.0 mL volumetric flask available to make this stock solution in. You are then tasked with using this stock solution to prepare a sample for use in an experiment. The experimental sample should have a final volume of 5.00 mL and a concentration of 0.150 M Mg(HCO3)2. a) What is the molar mass of Mg(HCO3)2? b) How many moles of the solute would be needed to create the stock solution using the volumetric flask available? c) How many grams of the solute should be added to the volumetric flask to create the stock solution? d) What volume, in mL, of the stock solution should be used to prepare the experimental solution through dilution?
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.16QAP
Related questions
Question
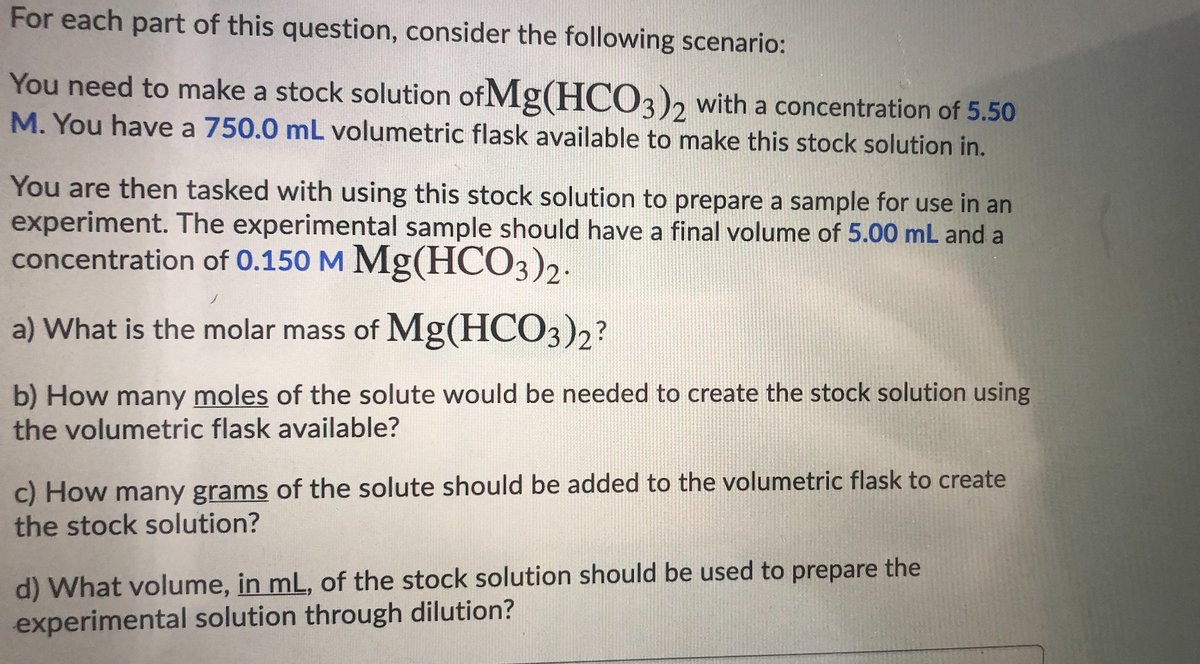
Transcribed Image Text:For each part of this question, consider the following scenario:
You need to make a stock solution of Mg(HC03)2 with a concentration of 5.50
M. You have a 750.0 mL volumetric flask available to make this stock solution in.
You are then tasked with using this stock solution to prepare a sample for use in an
experiment. The experimental sample should have a final volume of 5.00 mL and a
concentration of 0.150 M Mg(HCO3)2.
a) What is the molar mass of Mg(HCO3)2?
b) How many moles of the solute would be needed to create the stock solution using
the volumetric flask available?
c) How many grams of the solute should be added to the volumetric flask to create
the stock solution?
d) What volume, in mL, of the stock solution should be used to prepare the
experimental solution through dilution?
Expert Solution
Step 1
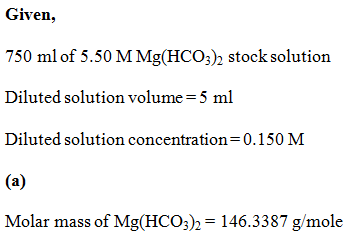
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

