b Answered: Sodium hydrogen cail A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdWKW_BBZZ16tTytly4Fcfu6zOtOf8oMM9sNvgu3hbeXU3orGsTW9r7w2eo0mgkaH6ew2mxV-VHO_rXdHb7LIF-UDKQ-zo?10BW7QY... Cameron - Cameron Hite - Learn D 3/5 O CHEMICAL REACTIONS Solving for a reactant in solution One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with iron(II) chloride, which would react with silver nitrate solution like this: FeCl,(aq) + 2 AgNO,(aq) → 2 AgCl(s) + Fe(NO,),(ac (be) The chemist adds 84.0 mM silver nitrate solution to the sample until silver chloride stops forming. She then washes, dries, and weighs the precipitate. She finds she has collected 2.3 mg of silver chloride. Calculate the concentration of iron(II) chloride contamiìnant in the original groundwater sample. Be sure your answer has the correct number of significant digits. Explanation Check ©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privac SMOTORZ BUR
b Answered: Sodium hydrogen cail A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdWKW_BBZZ16tTytly4Fcfu6zOtOf8oMM9sNvgu3hbeXU3orGsTW9r7w2eo0mgkaH6ew2mxV-VHO_rXdHb7LIF-UDKQ-zo?10BW7QY... Cameron - Cameron Hite - Learn D 3/5 O CHEMICAL REACTIONS Solving for a reactant in solution One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with iron(II) chloride, which would react with silver nitrate solution like this: FeCl,(aq) + 2 AgNO,(aq) → 2 AgCl(s) + Fe(NO,),(ac (be) The chemist adds 84.0 mM silver nitrate solution to the sample until silver chloride stops forming. She then washes, dries, and weighs the precipitate. She finds she has collected 2.3 mg of silver chloride. Calculate the concentration of iron(II) chloride contamiìnant in the original groundwater sample. Be sure your answer has the correct number of significant digits. Explanation Check ©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privac SMOTORZ BUR
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter7: Ionic Compounds And Metals
Section7.3: Names And Formulas For Ionic Compounds
Problem 39SSC
Related questions
Question
I need help solving for a reactant in a solution.
Transcribed Image Text:b Answered: Sodium hydrogen cail
A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdWKW_BBZZ16tTytly4Fcfu6zOtOf8oMM9sNvgu3hbeXU3orGsTW9r7w2eo0mgkaH6ew2mxV-VHO_rXdHb7LIF-UDKQ-zo?10BW7QY...
Cameron
- Cameron Hite - Learn
D 3/5
O CHEMICAL REACTIONS
Solving for a reactant in solution
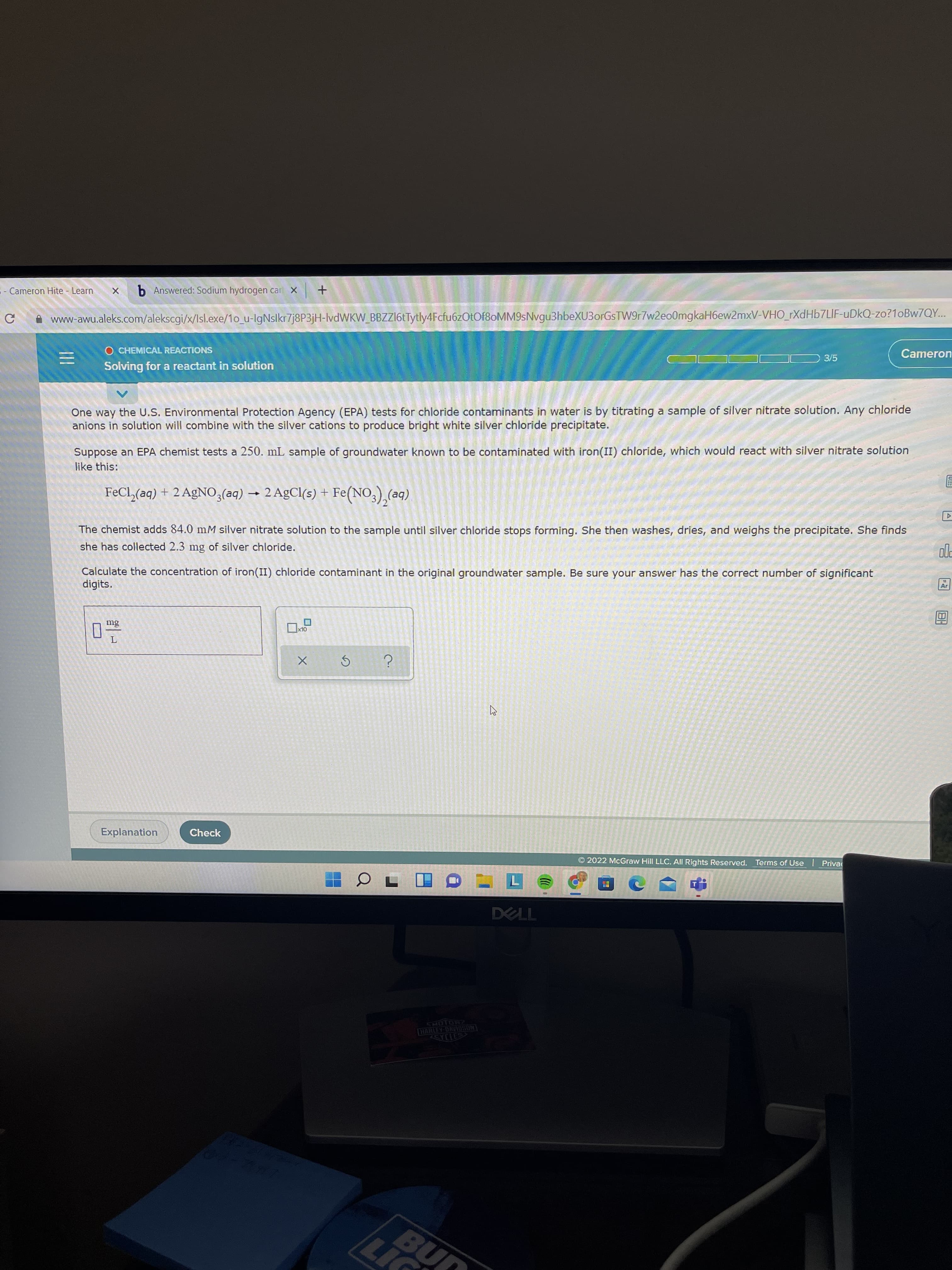
One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride
anions in solution will combine with the silver cations to produce bright white silver chloride precipitate.
Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with iron(II) chloride, which would react with silver nitrate solution
like this:
FeCl,(aq) + 2 AgNO,(aq) → 2 AgCl(s) + Fe(NO,),(ac
(be)
The chemist adds 84.0 mM silver nitrate solution to the sample until silver chloride stops forming. She then washes, dries, and weighs the precipitate. She finds
she has collected 2.3 mg of silver chloride.
Calculate the concentration of iron(II) chloride contamiìnant in the original groundwater sample. Be sure your answer has the correct number of significant
digits.
Explanation
Check
©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privac
SMOTORZ
BUR
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co