Consider the molecule OA || H;CA- CB – OBH 1. Indicate the hybridization employed by each central atom. CA : b. Св: а. OA : d. Ов: с. 2. Indicate the electron pair geometry about each central atom. CA : b. Св: а. OA : d. Ов: с. 3. Indicate the predicted bond angles around each central atom having more than one bond. CA : b. Св: Ов: а. с. 4. Describe all the o and t bonds in this molecule in the form of a hybridization and bonding scheme. For example, a hypothetical O-H bond = 0 (2 sp) – H (1 s). sigma bond(s) pi bond(s) 5. Which bond is the: а. shortest? b. strongest?
Consider the molecule OA || H;CA- CB – OBH 1. Indicate the hybridization employed by each central atom. CA : b. Св: а. OA : d. Ов: с. 2. Indicate the electron pair geometry about each central atom. CA : b. Св: а. OA : d. Ов: с. 3. Indicate the predicted bond angles around each central atom having more than one bond. CA : b. Св: Ов: а. с. 4. Describe all the o and t bonds in this molecule in the form of a hybridization and bonding scheme. For example, a hypothetical O-H bond = 0 (2 sp) – H (1 s). sigma bond(s) pi bond(s) 5. Which bond is the: а. shortest? b. strongest?
Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 87CP: Consider the following computer-generated model of caffeine: Complete a Lewis structure for caffeine...
Related questions
Question
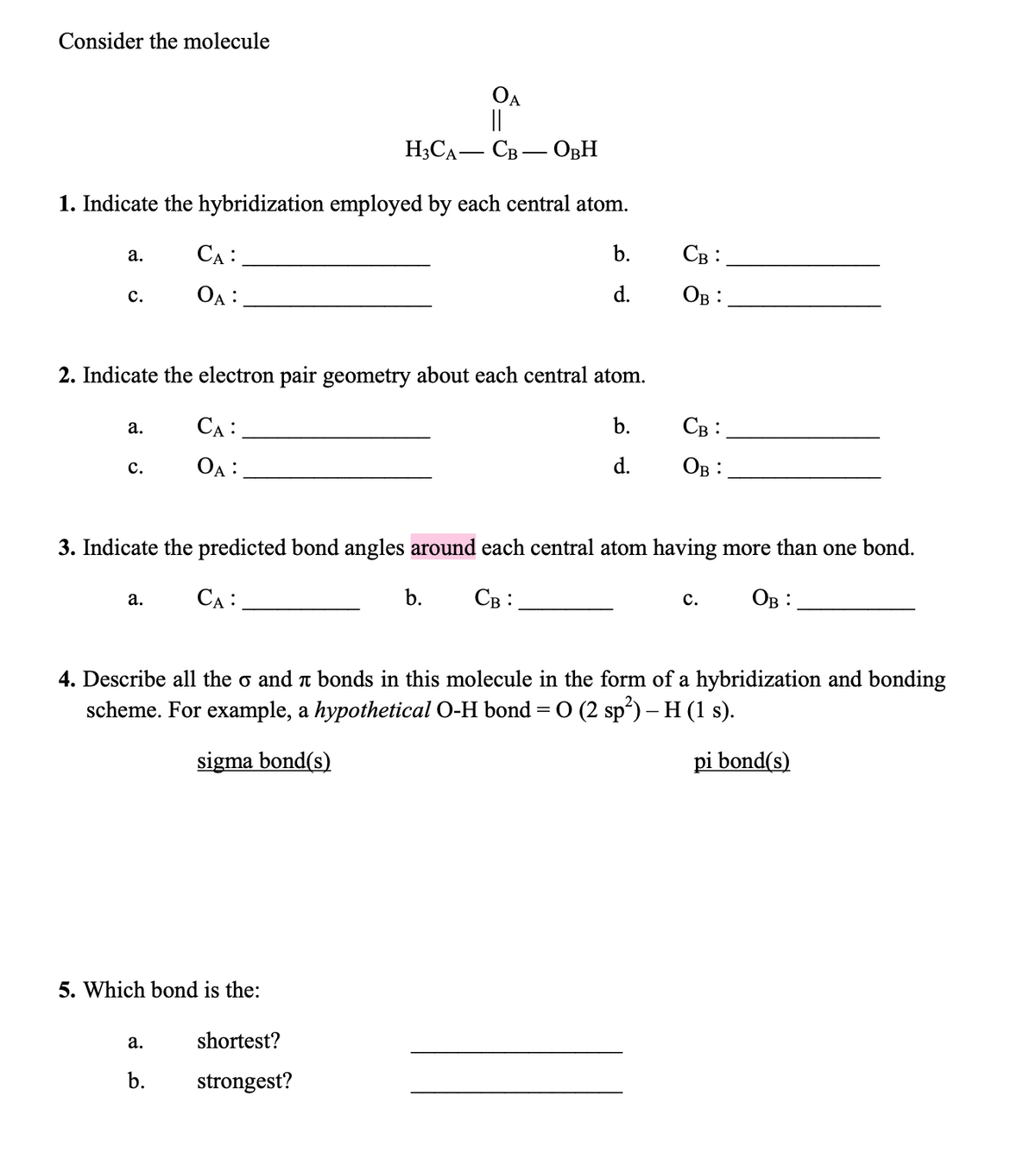
Transcribed Image Text:Consider the molecule
OA
||
H;CA- CB – OBH
1. Indicate the hybridization employed by each central atom.
CA :
b.
Св:
а.
OA :
d.
Ов:
с.
2. Indicate the electron pair geometry about each central atom.
CA :
b.
Св:
а.
OA :
d.
Ов:
с.
3. Indicate the predicted bond angles around each central atom having more than one bond.
CA :
b.
Св:
Ов:
а.
с.
4. Describe all the o and t bonds in this molecule in the form of a hybridization and bonding
scheme. For example, a hypothetical O-H bond = 0 (2 sp) – H (1 s).
sigma bond(s)
pi bond(s)
5. Which bond is the:
а.
shortest?
b.
strongest?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning