Table 2. Classification of Chemical Reactions Type of Reaction General Description and Example(s) Combination Two reactants combine to form a single product. The reactants may be elements or compounds. Also calied a synthesis reaction. Zn(s) + L(s) → Znl,(s) CaO(s) + H,O(1) → Ca(OH),(s) Decomposition One reactant, a compound, breaks down to give two or more products. 2H,0,(aq) → 2H,0(1) + 0,(g) Single Replacement An element reacts with a compound and replaces one of the elements in the compound. Metals replace hydrogen or other metals; nonmetals replace nonmetals. Zn(s) + 2HCI(aq) → H,(g) + ZNCI,(ag) Cu(s) + 2A£NO3(aq) → 2Ag(s) + Cu(NO,),(aq) Cl,(aq) + 2Nal(aq) L(aq} + 2NaCl(aq) Double Two ionic compounds (or compounds that break apart to form ions in solution) exchange ions to form new compounds. Examples include precipitation reactions (driving force is formation of a precipitate), acid–base reactions (driving force is formation of water), and gas-forming reactions (driving force is evolution of a gas). NaCl(aq) + A£NO3(aq) H,SO,(aq) + 2NaOH(aq) → Na,SO̟(aq) + 2H,0(1) Na,SO,(aq) + 2HCI(aq) Replacement A£CI(s) + NaNO3(aq) 2NAÇI(aq) + H,0(1) + SO,(g) Combustion A compound burns in the presence of oxygen, producing energy in the form of heat and light. The combustion of organic compounds produces carbon dioxide and water. C,H;(1) + 60,(g) → 4CO,(g) + 4H,0(g) b Questions 1. Write a balanced chemical equation for each reaction #1–8. Classify each reaction using the information provided in the Background section (see Table 2). Reaction #1: Mg (sy 029 Reaction #2: Mg RS Reaction #3: Culy)z COBLS)-) Reaction #4: Ca C0a 1s) Helcae? → Reaction #5: 2nis) + Cucizcae Reaction #6: Cucl caq? Nag Pou Log) > Reaction #7: H Cl cau) +Naotl cae Reaction #8: C 2Ho @lesOzug)> 2. Classifying chemical reactions helps chemists to predict the possible products that will ubetanees ava mived Complete and halance the following equa
Table 2. Classification of Chemical Reactions Type of Reaction General Description and Example(s) Combination Two reactants combine to form a single product. The reactants may be elements or compounds. Also calied a synthesis reaction. Zn(s) + L(s) → Znl,(s) CaO(s) + H,O(1) → Ca(OH),(s) Decomposition One reactant, a compound, breaks down to give two or more products. 2H,0,(aq) → 2H,0(1) + 0,(g) Single Replacement An element reacts with a compound and replaces one of the elements in the compound. Metals replace hydrogen or other metals; nonmetals replace nonmetals. Zn(s) + 2HCI(aq) → H,(g) + ZNCI,(ag) Cu(s) + 2A£NO3(aq) → 2Ag(s) + Cu(NO,),(aq) Cl,(aq) + 2Nal(aq) L(aq} + 2NaCl(aq) Double Two ionic compounds (or compounds that break apart to form ions in solution) exchange ions to form new compounds. Examples include precipitation reactions (driving force is formation of a precipitate), acid–base reactions (driving force is formation of water), and gas-forming reactions (driving force is evolution of a gas). NaCl(aq) + A£NO3(aq) H,SO,(aq) + 2NaOH(aq) → Na,SO̟(aq) + 2H,0(1) Na,SO,(aq) + 2HCI(aq) Replacement A£CI(s) + NaNO3(aq) 2NAÇI(aq) + H,0(1) + SO,(g) Combustion A compound burns in the presence of oxygen, producing energy in the form of heat and light. The combustion of organic compounds produces carbon dioxide and water. C,H;(1) + 60,(g) → 4CO,(g) + 4H,0(g) b Questions 1. Write a balanced chemical equation for each reaction #1–8. Classify each reaction using the information provided in the Background section (see Table 2). Reaction #1: Mg (sy 029 Reaction #2: Mg RS Reaction #3: Culy)z COBLS)-) Reaction #4: Ca C0a 1s) Helcae? → Reaction #5: 2nis) + Cucizcae Reaction #6: Cucl caq? Nag Pou Log) > Reaction #7: H Cl cau) +Naotl cae Reaction #8: C 2Ho @lesOzug)> 2. Classifying chemical reactions helps chemists to predict the possible products that will ubetanees ava mived Complete and halance the following equa
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter18: Oxidation–reduction Reactions And Electrochemistry
Section: Chapter Questions
Problem 6ALQ
Related questions
Question
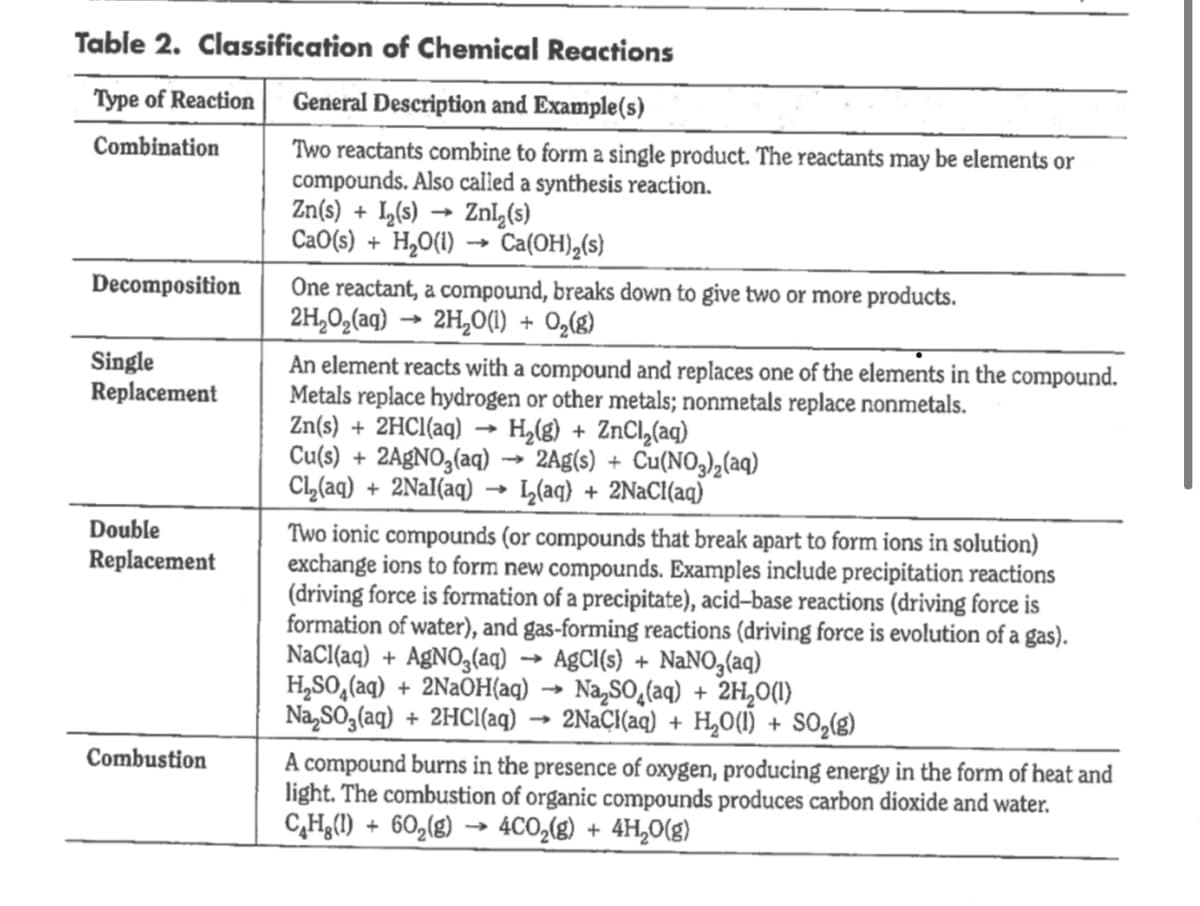
Transcribed Image Text:Table 2. Classification of Chemical Reactions
Type of Reaction
General Description and Example(s)
Combination
Two reactants combine to form a single product. The reactants may be elements or
compounds. Also calied a synthesis reaction.
Zn(s) + L(s) → Znl,(s)
CaO(s) + H,O(1) → Ca(OH),(s)
Decomposition
One reactant, a compound, breaks down to give two or more products.
2H,0,(aq) → 2H,0(1) + 0,(g)
Single
Replacement
An element reacts with a compound and replaces one of the elements in the compound.
Metals replace hydrogen or other metals; nonmetals replace nonmetals.
Zn(s) + 2HCI(aq) → H,(g) + ZNCI,(ag)
Cu(s) + 2A£NO3(aq) → 2Ag(s) + Cu(NO,),(aq)
Cl,(aq) + 2Nal(aq)
L(aq} + 2NaCl(aq)
Double
Two ionic compounds (or compounds that break apart to form ions in solution)
exchange ions to form new compounds. Examples include precipitation reactions
(driving force is formation of a precipitate), acid–base reactions (driving force is
formation of water), and gas-forming reactions (driving force is evolution of a gas).
NaCl(aq) + A£NO3(aq)
H,SO,(aq) + 2NaOH(aq) → Na,SO̟(aq) + 2H,0(1)
Na,SO,(aq) + 2HCI(aq)
Replacement
A£CI(s) + NaNO3(aq)
2NAÇI(aq) + H,0(1) + SO,(g)
Combustion
A compound burns in the presence of oxygen, producing energy in the form of heat and
light. The combustion of organic compounds produces carbon dioxide and water.
C,H;(1) + 60,(g) → 4CO,(g) + 4H,0(g)

Transcribed Image Text:b Questions
1. Write a balanced chemical equation for each reaction #1–8. Classify each reaction using
the information provided in the Background section (see Table 2).
Reaction #1: Mg (sy 029
Reaction #2: Mg RS
Reaction #3: Culy)z COBLS)-)
Reaction #4: Ca C0a 1s) Helcae? →
Reaction #5: 2nis) + Cucizcae
Reaction #6: Cucl caq? Nag Pou Log) >
Reaction #7: H Cl cau) +Naotl cae
Reaction #8: C 2Ho @lesOzug)>
2. Classifying chemical reactions helps chemists to predict the possible products that will
ubetanees ava mived Complete and halance the following equa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning