(8) A 8% Tab Window Help File Edit View History Bookmarks People b My Questions | bartleby Maryanne Dalton + x A ezto.mheducation.com/hm.tpx M Gmail YouTube Maps -3 -2 -1 +1 +2 +3 Rules for Assigning an Oxidation Number (O.N.) for Specific Atoms or Periodic Table Groups 1. For Group 1A(1): in l compounds = 'N'O 2. For Group 2A(2): O.N. = in all compounds 3. For hydrogen: O.N. = in combination with nonmetals O.N. = in combination with metals and boron 4. For fluorine: O.N. = in all compounds 5. For oxygen: O.N. = in peroxides O.N. = in all other compounds (except with F) 6. For Group 7A(17): in combination with metals, nonmetals (except O), and other halogens lower in the group. O.N. = Reset Zoom NOV 23 MacBook
(8) A 8% Tab Window Help File Edit View History Bookmarks People b My Questions | bartleby Maryanne Dalton + x A ezto.mheducation.com/hm.tpx M Gmail YouTube Maps -3 -2 -1 +1 +2 +3 Rules for Assigning an Oxidation Number (O.N.) for Specific Atoms or Periodic Table Groups 1. For Group 1A(1): in l compounds = 'N'O 2. For Group 2A(2): O.N. = in all compounds 3. For hydrogen: O.N. = in combination with nonmetals O.N. = in combination with metals and boron 4. For fluorine: O.N. = in all compounds 5. For oxygen: O.N. = in peroxides O.N. = in all other compounds (except with F) 6. For Group 7A(17): in combination with metals, nonmetals (except O), and other halogens lower in the group. O.N. = Reset Zoom NOV 23 MacBook
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 20Q
Related questions
Question
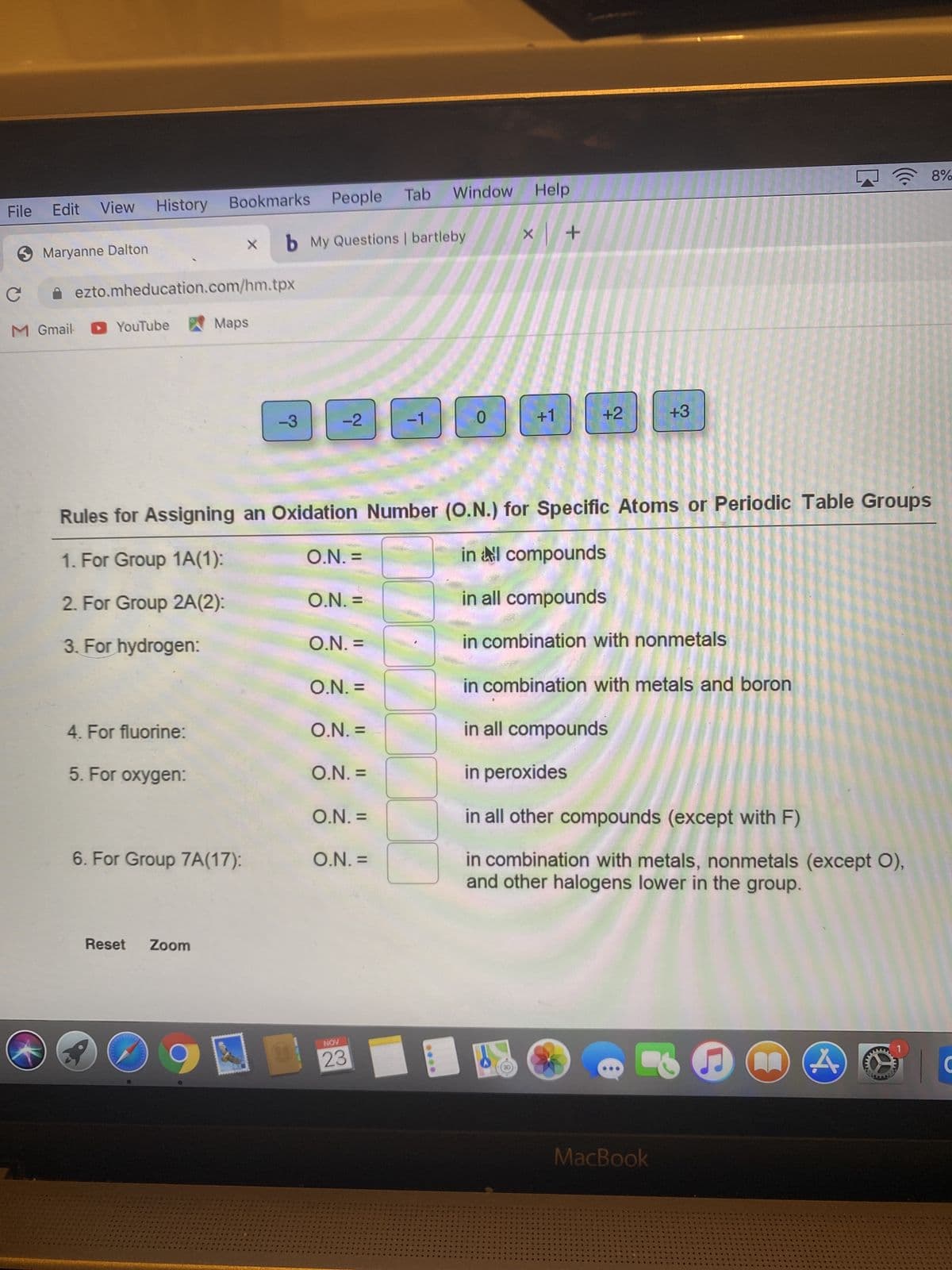
Transcribed Image Text:(8)
A 8%
Tab
Window Help
File
Edit
View
History Bookmarks People
b My Questions | bartleby
Maryanne Dalton
+ x
A ezto.mheducation.com/hm.tpx
M Gmail YouTube
Maps
-3
-2
-1
+1
+2
+3
Rules for Assigning an Oxidation Number (O.N.) for Specific Atoms or Periodic Table Groups
1. For Group 1A(1):
in l compounds
= 'N'O
2. For Group 2A(2):
O.N. =
in all compounds
3. For hydrogen:
O.N. =
in combination with nonmetals
O.N. =
in combination with metals and boron
4. For fluorine:
O.N. =
in all compounds
5. For oxygen:
O.N. =
in peroxides
O.N. =
in all other compounds (except with F)
6. For Group 7A(17):
in combination with metals, nonmetals (except O),
and other halogens lower in the group.
O.N. =
Reset
Zoom
NOV
23
MacBook
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning