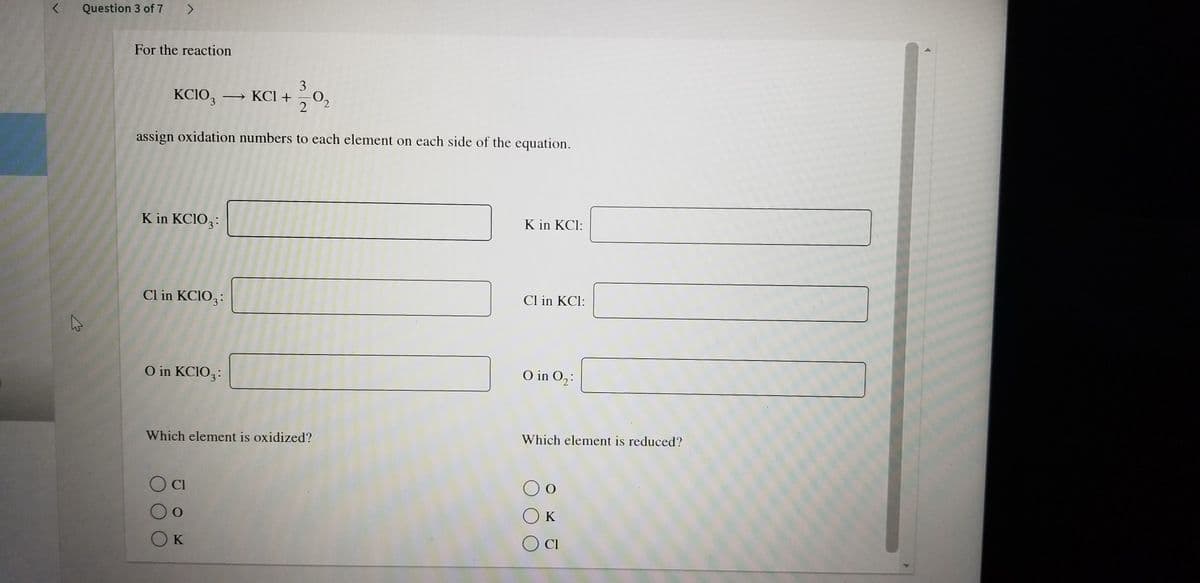
For the reaction KCIO, KCI +0, 2 assign oxidation numbers to each element on each side of the equation. K in KCIO,: K in KCI: Cl in KCIO,: Cl in KCI: O in KCIO,: O in O,: Which element is oxidized? Which element is reduced? OK
Q: Assign Oxidation number to each of the following and then label which element is going through…
A: Oxidation can be defined as the loss of electrons. E.g.- X: Any species. Reduction can be defined…
Q: What is the oxidation number of the underlined Hypothetical Element, X? I.) (NH4)2X(SO4)3 II.)…
A: We have to find the oxidation number of the underlined Hypothetical Element, X? I.) (NH4)2X(SO4)3…
Q: For the reaction 3 KCIO, - KCI + 0, 2 assign oxidation numbers to each element on each side of the…
A: Given-> KClO3 ----> KCl + 3/2(O2)
Q: 3. Use the changes in oxidation numbers to identify which atoms are oxidized and which are reduced…
A:
Q: For the reaction 3 KCIO3 KCI 2 assign oxidation numbers to each element on each side of the…
A: Oxidation state of each element of in KClO3, KCl and O2 is given,
Q: 2CrO42- + 3Pb+ 5H2O2Cr(OH)3 + 3HPbO2-+ OH- In the above redox reaction, use oxidation numbers to…
A: The substance whose oxidation increases in the reaction loses electrons. And since loss of electron…
Q: Balance the reactions below using the change in oxidation number method. Show complete solutions.…
A: The balanced equations are
Q: 8. – 10. In the chemical equation below, identify the elements that undergo changes in oxidation…
A: NaI(aq) + 3HOCl(aq) .....> NaIO3(aq) + 3HCl(aq) We know that: Usual ionic charge on H = +1…
Q: For the reaction KCIO → KCI + assign oxidation numbers to each element on each side of the equation.…
A: Oxidation means losss of electrons and reduction means gain of electrons.
Q: In the following reaction identify : 2K + MgCl2 ⟶⟶ 2KCl + Mg What is oxidized? What is reduced?…
A: In redox reaction oxidation and reduction takes place simultaneously in chemical reaction.
Q: For each of the following balanced equations, write the oxidation number above the symbol of each…
A: Since your question has multiple sub-parts, we will solve only first three sub-parts for you. If you…
Q: For the reaction KCIO → KCl + 0, 2 assign oxidation numbers to each element on each side of the…
A: The balanced chemical reaction given is KClO → KCl + 1/2 O2
Q: 10. What is the oxidizing and reducing agent in the following reaction? CO2(8) + H2(g) 2CO(g)…
A: in a reaction in which oxidation and reduction occurs simultaneously then that reaction is known as…
Q: Which species is the oxidizing agent in the following reaction? Mg + 2 HCl → MgCl2 + H2 Mg0 to…
A: Answer: H1+ to H0 An oxidising agent is the species that in a reaction oxidise other species by…
Q: Identify the oxidation number of the atom/s 1. NaCl 2. Cu2O 3. CaH2 4. S8 5. H2O2
A: We know that,. For neutral atom, some of oxidation number is equal to zero. If molecule present in…
Q: Balance the reactions below using the change in oxidation number method. PbO, + HBr → PbBr, + O, +…
A: From given 5 questions, first three questions are of same category and last 2 are of different…
Q: The following skeletal oxidation-reduction reaction occurs under basic conditions. Write the…
A:
Q: Cu2+ + 2Cr2+Cu + 2Cr3+ In the above redox reaction, use oxidation numbers to identify the element…
A: Oxidation: Loss of electronReduction: Gain of electronAn oxidizing agent gains the electrons and is…
Q: Pb + NiO2+ H2O+ OH- ---> HPbO2- + Ni(OH)2 In the above redox reaction, use oxidation…
A: Given:
Q: HPbO2- + H2PO2-Pb + HPO32-+ H2O In the above redox reaction, use oxidation numbers to identify the…
A: The reaction taking place is given as, => HPbO2- + H2PO2- --------> Pb + HPO32- + H2O
Q: 6HC10 + 2NO →3Cl, + 2HNO3+ 2H,O In the above redox reaction, use oxidation numbers to identify the…
A: Reductin reacton = addition of the electron or reduction of oxidation number. Oxidation reaction =…
Q: Bi + Mno, + 2H,0 Mno2 + Bi(OH)3 + OH- For the above redox reaction, assign oxidation numbers and use…
A: The given reaction is : The oxidation number of O is -2 The oxidation number of H is +1.
Q: For the reaction KCIO, КСІ + 0, assign oxidation numbers to each element on each side of the…
A: The given reaction is as follows: KClO2 → KCl + O2 The oxidation of an element is defined as the…
Q: For the reaction KCIO – KCI + 0, assign oxidation numbers to each element on each side of the…
A:
Q: Determine whether the underlined element is oxidized or reduced in the given partial reaction. 2. a)…
A:
Q: Balance the equation and choose the coefficient of the (?) Na2CO3 + HCl → NaCl + H20 + CO2 (?…
A: Given that : We have to balance the following equation and choose the coefficient of the (?) :…
Q: what is the oxidation numbers of : cu (s) + hno3(aq) --> cu(no3)2(aq) + 2 no2 (g) in table format
A:
Q: In the following reaction, which is the oxidizing agent? AgNO2+ Cl₂ + 2KOH → AgNO3+2KCI + H₂O O…
A: Answer : Correct option is (b) Cl2 Explainination : oxidizing agent is a…
Q: Balance the following reactions using oxidation number method. Bi3+(aq) + Mg(s) → Bi(s) + Mg2+(aq)
A: Given Bi3+(aq) + Mg(s) → Bi(s) + Mg2+(aq)
Q: Determine the oxidation number (oxidation state) of each element in the compound CUCO,. Cu: +3 C:…
A: "To get remaining sub-parts solved please repost the complete question and mention the sub-parts to…
Q: Balance the reactions below using the change in oxidation number method. РЬО, + НВr РЬО + NH, 1.…
A: Since you have posted multiple questions according to guidelines I am solving first three.If you…
Q: Which element is oxidized and which is reduced in the following reactions? N2(g) + 3H2(g) -->…
A: Redox reaction: A reaction in which both oxidation and reduction takes place and oxidation states of…
Q: Use the oxidation states method to balance the reaction: __ SO42– (aq) + __ Fe2+ (aq) +…
A:
Q: 6I- + 2Cr(OH)33I2 + 2Cr+ 6OH- In the above redox reaction, use oxidation numbers to identify the…
A: Increase in oxidation no. Is oxidation & decrease in oxidation no. Is reduction. Compound is…
Q: Balance the reactions below using the change in oxidation number method. PbO2 + HBr → PbBr, + O, +…
A:
Q: Balance by change in oxidation number KMnO4 + NaCl + H2SO4 —-> MnSO4 + Na2SO4 + K2SO4 + Cl2 + H2O
A: In this question, we have to find out the correct answer of given problem by the help of the…
Q: Q: Calculate the BOD of a water sample containing 300 mg of phenol in one liter of water?…
A: BOD (Biological Oxygen Demand) is the oxygen required by the bio-degradable organic matter present…
Q: = whether each chemical reaction in the table below is an oxidation-reduction ("red reducing agent…
A:
Q: 6) For the following redox reaction: Fe203(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g) a) What is the…
A:
Q: Balance the following reactions using oxidation number method. Bi3+(aq) + Mg(s) ® Bi(s) + Mg2+(aq)
A: Given: Bi3+(aq) + Mg(s) → Bi(s) + Mg2+(aq)
Q: Assign oxidation numbers to the underlined element. 1) K 2)…
A: Oxidation number: The covalent bond between the atoms of different electronegativity is polar. If we…
Q: Balance the reactions below using the change in oxidation number method. KBr + KClO2+ H2SO4 → Br2 +…
A: Note: Since you have asked multiple questions, we will solve the first for you. If you want any…
Q: Determine the Oxidation Numbers of each element in the following substances: a. Li,o Li: 0: b. Cr,0,…
A: “Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: Which of the following has the highest (more positive) oxidation number? Al(s) H in water O O in…
A: Given that : We have to determine which of the following has the highest oxidation number :
Q: Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox")…
A: The species which gains electron and reduced in a reaction is known as oxidizing agent. The species…
Q: Which of the following has the highest (more positive) oxidation number? All(s) O in water H in…
A: Which of the following has the highest (more positive) oxidation number?
Q: Which of the following reactions would NOT be an oxidation/reduction or Redox reaction? Ca(OH)2 +2…
A: A redox reaction is a type of chemical reaction which involves the movement of electrons between two…
Q: Use the oxidation states method to balance the reaction: __ SO42– (aq) + __ Fe2+ (aq) +…
A: Applying concept of balancing chemical reaction.
Q: Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent…
A: Oxidation :- The process of loss of electrons or increase in oxidation number is known as oxidation…
Q: For the reaction 3 → KCI + ¬0, KCIO, assign oxidation numbers to each element on each side of the…
A: Given reaction is KClO3 ——> KCl + 3/2 O2 We need to find oxidation states of elements in…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- 4-28 Answer true or false. (a) A net ionic equation shows only those ions that undergo chemical reaction. (b) In a net ionic equation, the number of moles of starting material must equal the number of moles of product. (c) A net ionic equation must be balanced by both mass and charge. (d) As a generalization, all lithium, sodium, and potassium salts are soluble in water. (e) As a generalization, all nitrate (NO3- salts are soluble in water. (f) As a generalization, most carbonate (CO3) salts are insoluble in water. (g) Sodium carbonate, Na2CO3, is insoluble in water. (h) Ammonium carbonate, (NH4 )2CO3, is insoluble in water. (j) Calcium carbonate, CaCO3, is insoluble in water. (j) Sodium dihydrogen phosphate, NaH2PO4, is insoluble in water. (k) Sodium hydroxide, NaOH, is soluble in water. (l) Barium hydroxide, Ba(OH)2, is soluble in water.Calculate the oxidation number of the underlined element. a) HClO4 b) CH4 c) PO4 3- Consider the photosynthesis reaction: CO2 + H2O → CH2O + O2 Change in O.N. for oxygen: ????1, Find the oxidation numbers of the elements in bold print. a) HClO d) PbSO4 g) Na2O2 b) KClO3 e) NaIO4 h) K2SO4 c) MnO2 f) ClO41- i) NH41+ 2, State whether the change is an oxidation or a reduction. a) MnO41– becomes MnO42– __________d) P4O6 becomes P4O10 ____________ b) N2 becomes NH3 ____________ e) NH3 becomes N2O ____________ c) O2- becomes O2 ____________ f) SO42– becomes S2O32– ____________
- 3. a) When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?Mg2+ + Mn2+ Mg + MnO4- Water appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.)How many electrons are transferred in this reaction? b) When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?Cl2 + Zn2+HClO + ZnWater appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.)How many electrons are transferred in this reaction? c) When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?Zn + HNO3 Zn2+ + NO Water appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.)How many electrons are transferred in…2CrO42- + 3Pb+ 5H2O2Cr(OH)3 + 3HPbO2-+ OH- In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent.When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown?Cl- + NiO2 Ni(OH)2 + Cl2 Water appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.)Which element is oxidized?
- a. What is the molarity of a solution of KMnO4 if 40.00 ml will oxidize 0.3 g Na2C2O4 (134 g/mol)? b. What is the value of 1.00 ml of the above solution in terms of FeSO4.7H2O ( 278 g/mol) c. What is the value of 1.00 ml of the above permanganate solution in terms of grams As2O3 (198 g/mol)Combustion reactions (burning of fuels) are another example of redox. Coal is nearly pure carbon and has been burned as a fuel for centuries. The reaction of coal with oxygen in the air is illustrated by the balanced equation: C + O2 → CO2 What is the change in oxidation state for the carbon in this reaction? Group of answer choices +2 -4 +4How to write net iconic reaction of Al2(SO4)3(NH4)SO4•24H2O + BaCl --> ?
- When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown?Pb + Cr2O72- ---->Pb2+ + Cr3+Water appears in the balanced equation as a ____ (reactant, product, neither) with a coefficient of ____(Enter 0 for neither.) Which species is the oxidizing agent?13. Balance the following reaction under basic conditions. What are the coefficients in front of H2O and Cl- in the balanced reaction? Cl2(aq) + Br2(l) → BrO3-(aq) + Cl-(aq) Group of answer choices A) H2O = 2, Cl- = 2 B) H2O = 2, Cl- = 5 C) H2O = 6, Cl- = 10 D) H2O = 4, Cl- = 6 E) H2O = 7, Cl- = 3When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?________Ag+ +_______ Mg------->_____Ag + ____Mg2+Water appears in the balanced equation as a _______ (reactant, product, neither) with a coefficient of__________ . (Enter 0 for neither.)Which element is oxidized? ___________

