
IUPAC nomenclature-
1) Number the longest carbon chain.
2) Numbering starts from double bond in alkene, triple bond in alkyne.
3) For substituted compounds, first substituent must be lowest locator.
4) Prefix such as di,tri ,tetra are used for number of groups.
5)Use (-) between number and alphabets
6) use (,) between numbers.
7) Use suffix ane for alkyne , ene for alkene and yne for alkyne , cyclo for cyclic compound along with parent compound.
a)
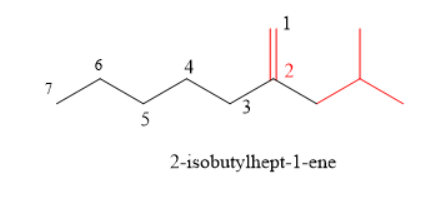
Longest chain is of 7 carbons. Therefore parent compound = heptane
Give numbering from that double bonded carbon.
There is iso-butly group attached to carbon number 2
Double bond is between C1 and C2
Naming is done in alphabetical order.
This compound is alkene so use suffix ene.
IUPAC name is 2-isobutylhept-1-ene.
b)
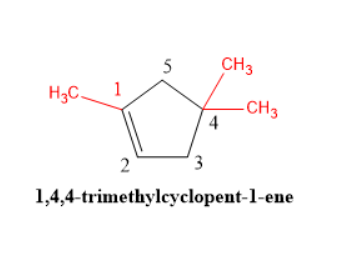
This is cyclic compound. Use prefix ' Cyclo'
Numbering from double bonded carbon.
Double bond is between C1-C2
Total number of carbon = 5 parent compound is pentane
This is alkene.
Therefore, pent-1-ene
There are three methyl substituents. one at carbon number 1 and 2 at carbon number number 4.
Use suffix trimethyl
IUPAC name of compound is 1,4,4-trimethylcyclpoent-1-ene
Step by step
Solved in 6 steps with 9 images









