Data and Minor Calculations Part I standardization of the sodium hydroxide Table 1 : Titration of 25.00 mL of standard sulfuric acid solution with sodium hydroxide Trial Initial burc reading (ml) 0.52 Final buret reading (m²) Volume of sodium hydroxide (ML) Part 2. Analysir of unknown Table 2 Titration with Initial buret reading (ml) Final buret reading (ml) 47.83 Volume of sodium hydroxide (ml) 37.54 37.55 Tared mass of unknown sample : 1.5289 #137256 Concentration of standard sulfuric acid solution : 0.04953 12. Calculate the mass of sodium carbonate present in the original 250.0 mL of solution. 13. Calculate the percent of sodium carbonate in the impure sample. 24-92 24.40 Carbonate of impure sodium carbonate Tri al sodium hydroxide solution 10.51 38.05 Solution 2 2 25 18.35 44.78 24.43 10.28
Data and Minor Calculations Part I standardization of the sodium hydroxide Table 1 : Titration of 25.00 mL of standard sulfuric acid solution with sodium hydroxide Trial Initial burc reading (ml) 0.52 Final buret reading (m²) Volume of sodium hydroxide (ML) Part 2. Analysir of unknown Table 2 Titration with Initial buret reading (ml) Final buret reading (ml) 47.83 Volume of sodium hydroxide (ml) 37.54 37.55 Tared mass of unknown sample : 1.5289 #137256 Concentration of standard sulfuric acid solution : 0.04953 12. Calculate the mass of sodium carbonate present in the original 250.0 mL of solution. 13. Calculate the percent of sodium carbonate in the impure sample. 24-92 24.40 Carbonate of impure sodium carbonate Tri al sodium hydroxide solution 10.51 38.05 Solution 2 2 25 18.35 44.78 24.43 10.28
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.40QAP
Related questions
Question
Typewritten for an upvote. No upvote for handwritten. Please skip if you have already done this. Thank you
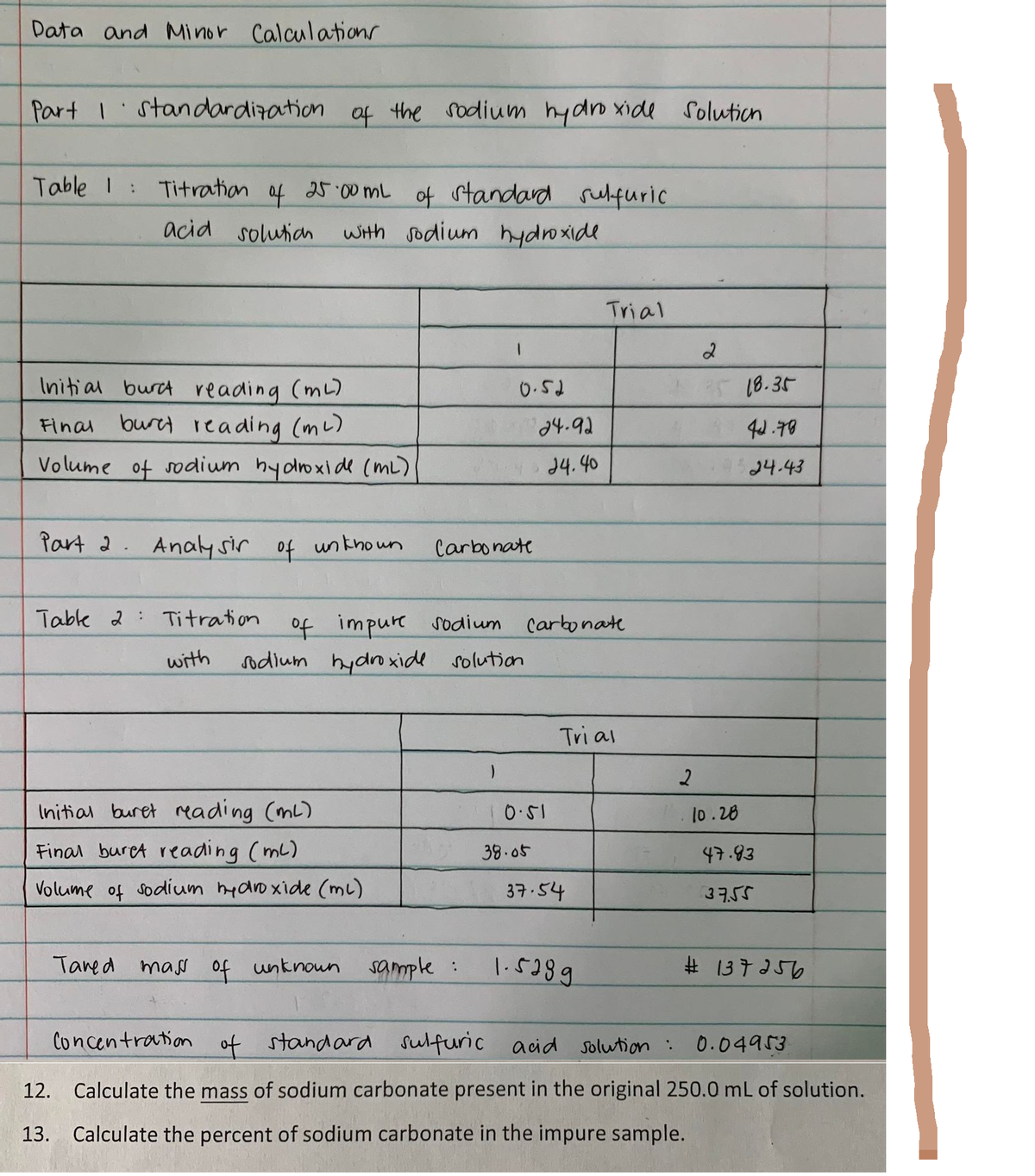
Transcribed Image Text:Data and Minor Calculations
Part I standardization of the sodium hydroxide
Table 1 :
Titration
of
25.00 mL
of standard sulfuric
acid solution with sodium hydroxide
Trial
Initial burc reading (ml)
0.52
Final buret reading (m²)
Volume of sodium hydroxide (ML)
Part 2. Analysir of unknown
Table 2 Titration
with
Initial buret reading (ml)
Final buret reading (ml)
47.83
Volume of sodium hydroxide (ml)
37.54
37.55
Tared mass of unknown
sample :
1.5289
#137256
Concentration of standard sulfuric acid solution :
0.04953
12. Calculate the mass of sodium carbonate present in the original 250.0 mL of solution.
13. Calculate the percent of sodium carbonate in the impure sample.
24-92
24.40
Carbonate
of impure sodium carbonate
Tri al
sodium hydroxide solution
10.51
38.05
Solution
2
2
25 18.35
44.78
24.43
10.28
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
