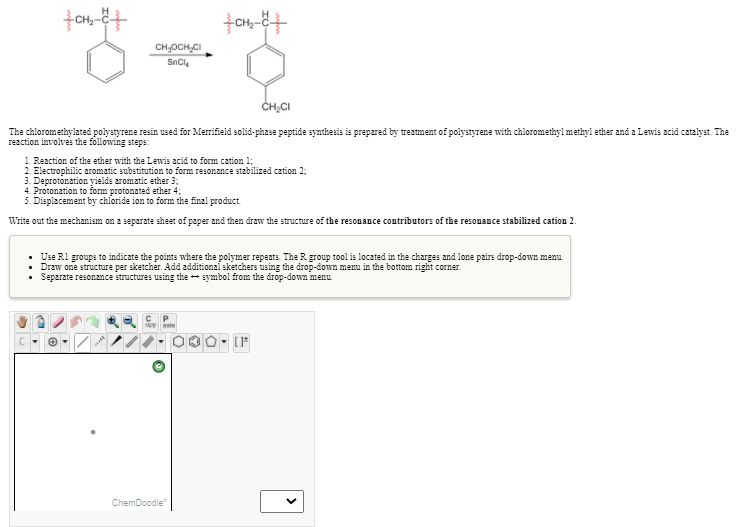
CH,OCH,CI SnCl, CH,CI The chloromethylated polystyrene resin used for Merrifield solid-phase peptide synthesis is prepared by treatment of polystyrene with chloromethylmethyl ether and a Lewis acid catalyst. The reaction involves the following steps: 1. Reaction of the ether with the Lewis acid to form cation 1; 2. Electrophilic aromatic substitution to form resonance stzbilized cation 2: 3. Deprotonation yields aromatic ether 3: 4. Protonation to žorm protonated ether 4; 5. Displacement by chloride ion to form the final product Write out the mechanism on a separate sheet of paper and then draw the structure of the resonance contributors of the resonance stabilized cation 2. • Use Rl groups to indicate the points where the polymer repeats. The R group tool is located in the charges and lone pairs drop-down menu. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate resonance structures using the symbol from the drop-down menu - [F
CH,OCH,CI SnCl, CH,CI The chloromethylated polystyrene resin used for Merrifield solid-phase peptide synthesis is prepared by treatment of polystyrene with chloromethylmethyl ether and a Lewis acid catalyst. The reaction involves the following steps: 1. Reaction of the ether with the Lewis acid to form cation 1; 2. Electrophilic aromatic substitution to form resonance stzbilized cation 2: 3. Deprotonation yields aromatic ether 3: 4. Protonation to žorm protonated ether 4; 5. Displacement by chloride ion to form the final product Write out the mechanism on a separate sheet of paper and then draw the structure of the resonance contributors of the resonance stabilized cation 2. • Use Rl groups to indicate the points where the polymer repeats. The R group tool is located in the charges and lone pairs drop-down menu. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate resonance structures using the symbol from the drop-down menu - [F
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 63AP: As far back as the 16th century, South American Incas chewed the leaves of the coca bush,...
Related questions
Question
Transcribed Image Text:CH,OCH,CI
SnCl,
CH,CI
The chloromethylated polystyrene resin used for Merrifield solid-phase peptide synthesis is prepared by treatment of polystyrene with chloromethylmethyl ether and a Lewis acid catalyst. The
reaction involves the following steps:
1. Reaction of the ether with the Lewis acid to form cation 1;
2. Electrophilic aromatic substitution to form resonance stzbilized cation 2:
3. Deprotonation yields aromatic ether 3:
4. Protonation to žorm protonated ether 4;
5. Displacement by chloride ion to form the final product
Write out the mechanism on a separate sheet of paper and then draw the structure of the resonance contributors of the resonance stabilized cation 2.
• Use Rl groups to indicate the points where the polymer repeats. The R group tool is located in the charges and lone pairs drop-down menu.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
• Separate resonance structures using the symbol from the drop-down menu
ChemDoodle"
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning