Choose a ratio showing the relationship between the amounts of each element for each of the following. (Blue balls represent nitrogen atoms, red balls represent oxygen atoms, white balls represent hydrogen atoms, black balls represent carbon atoms, and yellow balls represent sulfur atoms.) O:S 90:3S 30:S 0:3S
Choose a ratio showing the relationship between the amounts of each element for each of the following. (Blue balls represent nitrogen atoms, red balls represent oxygen atoms, white balls represent hydrogen atoms, black balls represent carbon atoms, and yellow balls represent sulfur atoms.) O:S 90:3S 30:S 0:3S
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 1CR
Related questions
Question
Please answer question 15 Part A and B
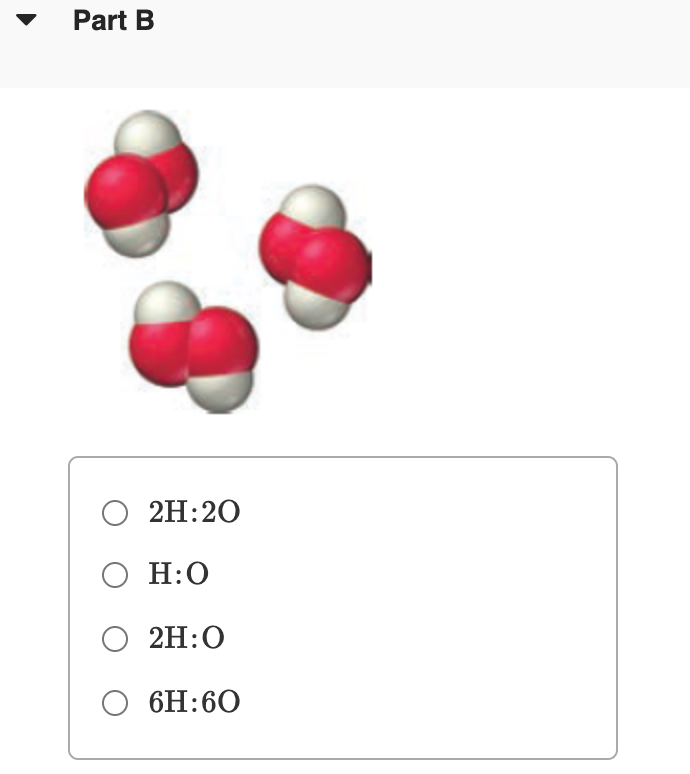
Transcribed Image Text:Part B
O 2H:20
O H:0
O 2H:0
O 6H:60
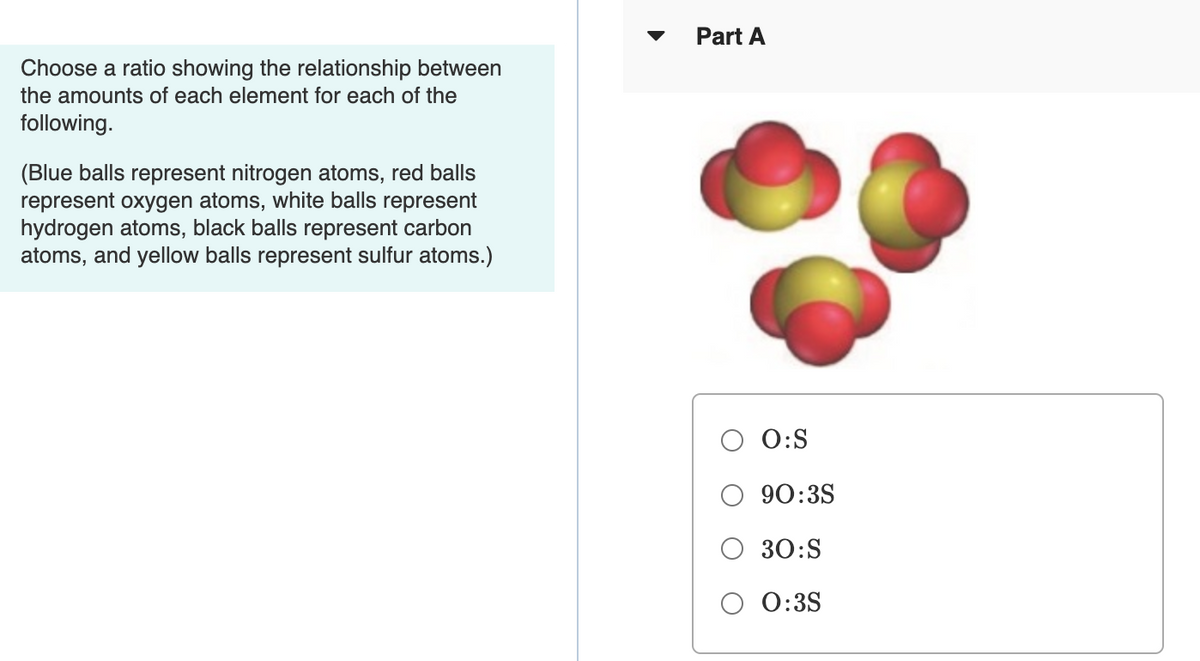
Transcribed Image Text:Part A
Choose a ratio showing the relationship between
the amounts of each element for each of the
following.
(Blue balls represent nitrogen atoms, red balls
represent oxygen atoms, white balls represent
hydrogen atoms, black balls represent carbon
atoms, and yellow balls represent sulfur atoms.)
O:S
90:3S
30:S
0:3S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning