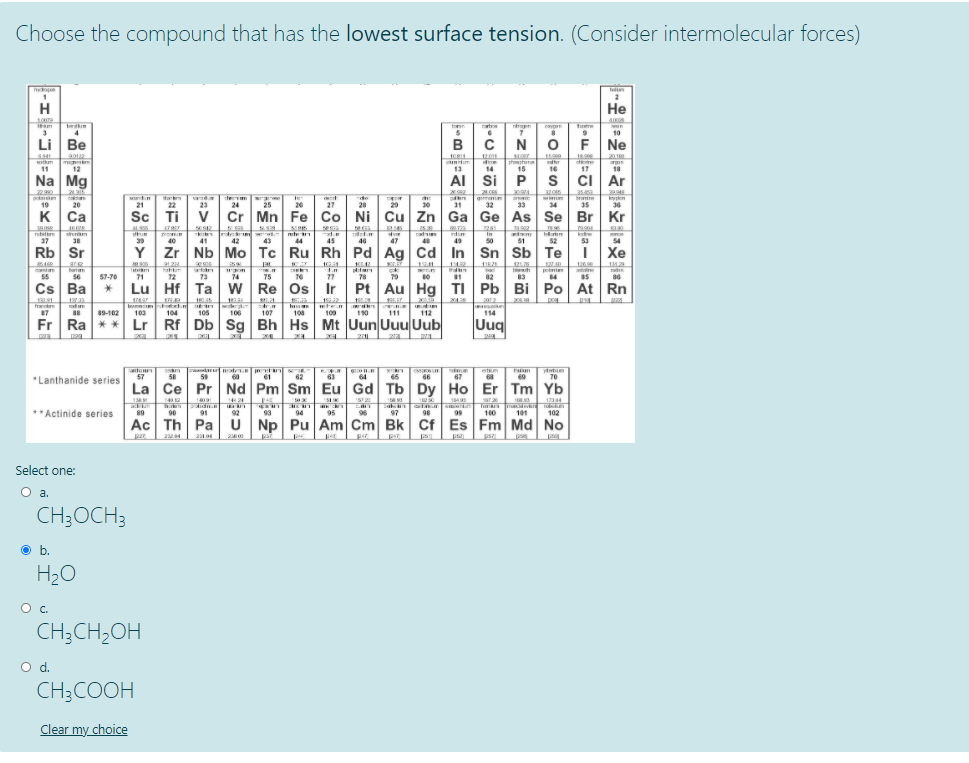
Choose the compound that has the lowest surface tension. (Consider intermolecular forces) 2 H Не 400 am ate rgen tere 10 Li Be N F Ne 1201 thstera 15 11 12 13 14 16 17 18 Na Mg AI Si S ci Ar 32.010 नता tan de nire 35 gimnie 19 20 21 22 23 24 25 26 27 28 20 30 31 32 33 34 36 к Са Sc Ti Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 241 shen nite In wwe 37 38 39 40 41 42 43 44 45 47 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Те Xe KLA 12 T n 71 barim plme 55 56 57-70 12 73 74 75 77 78 79 02 Cs Ba Lu Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn IEL 20 87 19-102 103 104 105 106 107 108 100 110 111 112 114 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq etn 57 58 59 62 63 66 67 69 70 "Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 1424 tex BEHT 134 Ta 97 Tu 100 **Actinide series 89 90 91 92 93 94 95 98 99 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Select one: O a CH;OCH3 O b. H20 CH;CH;OH d. CH3COOH Clear my choice
Choose the compound that has the lowest surface tension. (Consider intermolecular forces) 2 H Не 400 am ate rgen tere 10 Li Be N F Ne 1201 thstera 15 11 12 13 14 16 17 18 Na Mg AI Si S ci Ar 32.010 नता tan de nire 35 gimnie 19 20 21 22 23 24 25 26 27 28 20 30 31 32 33 34 36 к Са Sc Ti Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 241 shen nite In wwe 37 38 39 40 41 42 43 44 45 47 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Те Xe KLA 12 T n 71 barim plme 55 56 57-70 12 73 74 75 77 78 79 02 Cs Ba Lu Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn IEL 20 87 19-102 103 104 105 106 107 108 100 110 111 112 114 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq etn 57 58 59 62 63 66 67 69 70 "Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 1424 tex BEHT 134 Ta 97 Tu 100 **Actinide series 89 90 91 92 93 94 95 98 99 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Select one: O a CH;OCH3 O b. H20 CH;CH;OH d. CH3COOH Clear my choice
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 121CWP: Which of the following compound(s) exhibit only London dispersion intermolecular forces? Which...
Related questions
Question
Transcribed Image Text:Choose the compound that has the lowest surface tension. (Consider intermolecular forces)
Не
Iam
rgen
Tutre
wen
4
10
Li Be
B
N
F Ne
1201
15.00
201
otm
11
phster
15
13
AI Si P
12
14
16
17
18
Na Mg
s ci Ar
354
pte
de
n
36
gmenim
bmire
25
32 33
19
20
21
22
23
24
26
27
28
29
30
31
34
35
K
к Са
Ca
Sc Ti
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
261
ndim
In
37
38
40
41
42
43
44
45
46
47
49
50
51
52
53
54
Rb Sr
Y
Zr |Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te
i Xe
12125
126.0
hatm
71
79
ponim
84
85
55
56
57-70
72
73
74
75
77
78
82
83
Cs Ba
Lu Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
120.91
20
In
19-102 103 104
114
Uuq
87
105
106
107
108
10010 12
111
Fr
Ra
** Lr Rf Db Sg Bh Hs Mt Uun UuuUub
etn
68
58
60
61
62
64
65
66
67
70
"Lanthanide series
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
1409
14424
1234
**Actinide series
Tu
10 101102
89
90
91
92
93
94
95
96
97
98
99
Ac Th Pa ü
Np Pu Am Cm Bk Cf Es Fm Md No
us L
224
Select one:
Oa.
O a.
CH;OCH3
Ob.
O b.
H20
Oc.
CH;CH2OH
d.
CH3COOH
Clear my choice
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning