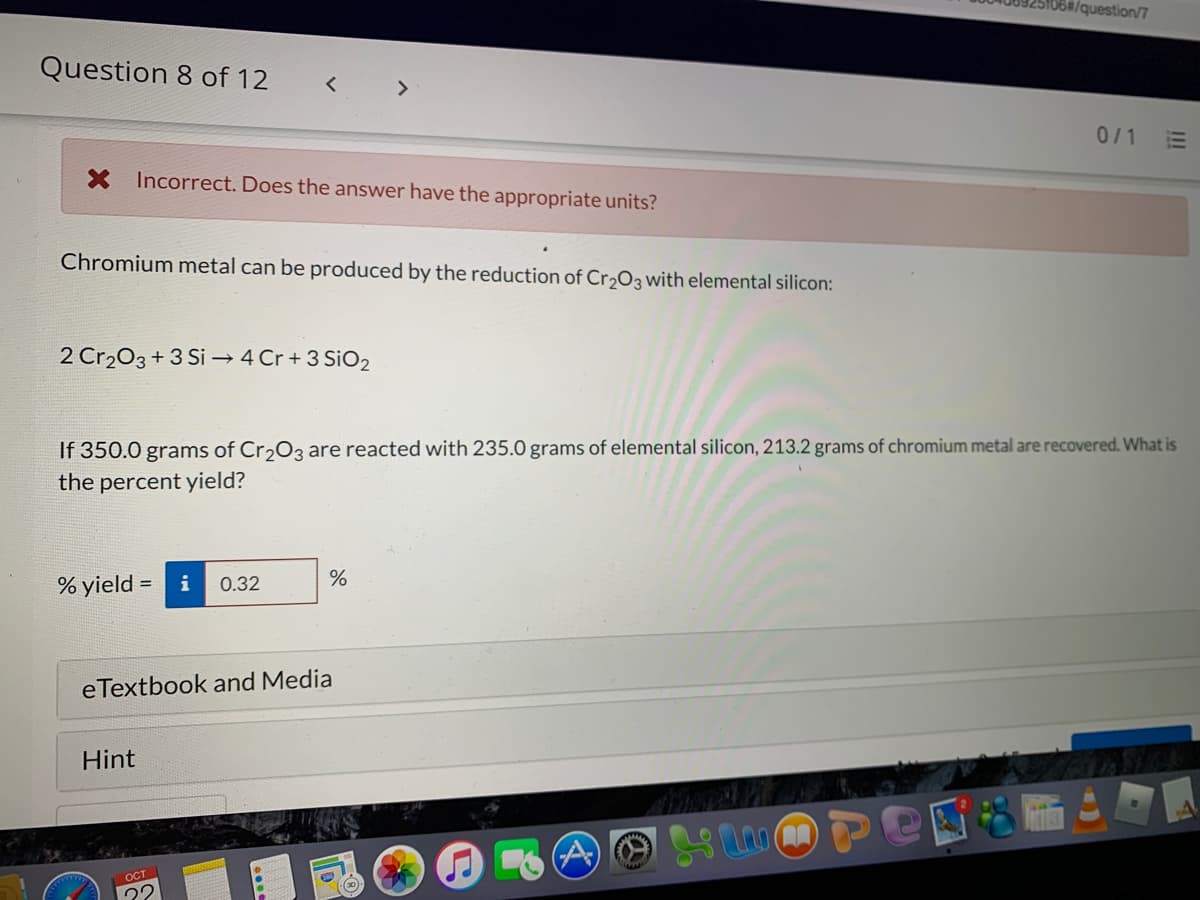
Chromium metal can be produced by the reduction of Cr2O3 with elemental silicon: 2 Cr203 + 3 Si → 4 Cr + 3 SiO2 If 350.0 grams of Cr203 are reacted with 235.0 grams of elemental silicon, 213.2 grams of chromium metal are recovered. What is the percent yield? % yield = i 0.32
Chromium metal can be produced by the reduction of Cr2O3 with elemental silicon: 2 Cr203 + 3 Si → 4 Cr + 3 SiO2 If 350.0 grams of Cr203 are reacted with 235.0 grams of elemental silicon, 213.2 grams of chromium metal are recovered. What is the percent yield? % yield = i 0.32
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.27QAP
Related questions
Question
Transcribed Image Text:/question/7
Question 8 of 12
< >
0/1
!!
X Incorrect. Does the answer have the appropriate units?
Chromium metal can be produced by the reduction of Cr2O3 with elemental silicon:
2 Cr203+3 Si → 4 Cr + 3 SiO2
If 350.0 grams of Cr,O3 are reacted with 235.0 grams of elemental silicon, 213.2 grams of chromium metal are recovered. What is
the percent yield?
% yield = i
0.32
eTextbook and Media
Hint
Ост
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

