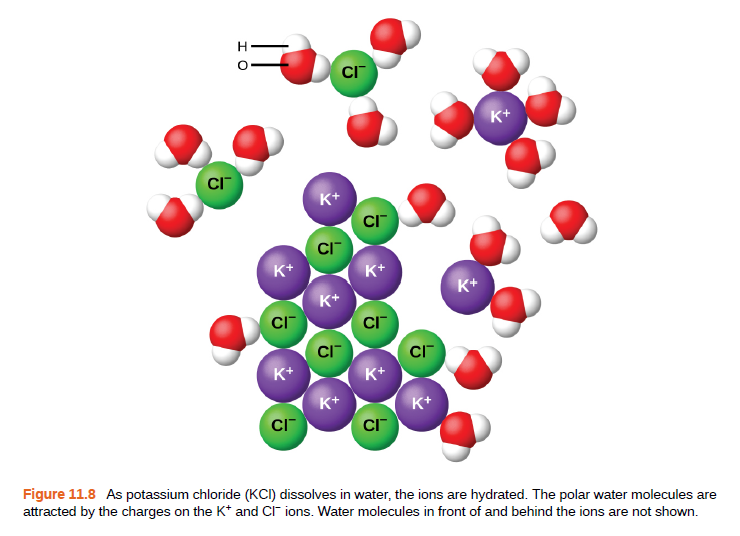
CI K+ K+ CI K* K+ K+ K+ CI CI CI CI K+ K+ K+ K+ CI CI Figure 11.8 As potassium chloride (KCI) dissolves in water, the ions are hydrated. The polar water molecules are attracted by the charges on the K* and CI ions. Water molecules in front of and behind the ions are not shown. I O
CI K+ K+ CI K* K+ K+ K+ CI CI CI CI K+ K+ K+ K+ CI CI Figure 11.8 As potassium chloride (KCI) dissolves in water, the ions are hydrated. The polar water molecules are attracted by the charges on the K* and CI ions. Water molecules in front of and behind the ions are not shown. I O
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 56QRT
Related questions
Question
Indicate the most important type of intermolecular attraction responsible for solvation in each of the following solutions:
(a) the solutions as shown
(b) methanol, CH3OH, dissolved in ethanol, C2H5OH
(c) methane, CH4, dissolved in benzene, C6H6
(d) the polar halocarbon CF2Cl2 dissolved in the polar halocarbon CF2ClCFCl2
(e) O2(l) in N2(l)
Transcribed Image Text:CI
K+
K+
CI
K*
K+
K+
K+
CI
CI
CI
CI
K+
K+
K+
K+
CI
CI
Figure 11.8 As potassium chloride (KCI) dissolves in water, the ions are hydrated. The polar water molecules are
attracted by the charges on the K* and CI ions. Water molecules in front of and behind the ions are not shown.
I O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning