Chapter3: Mechanisms
Section: Chapter Questions
Problem 88EQ
Related questions
Question
Draw the products of attached nucleophilic substitution reaction.
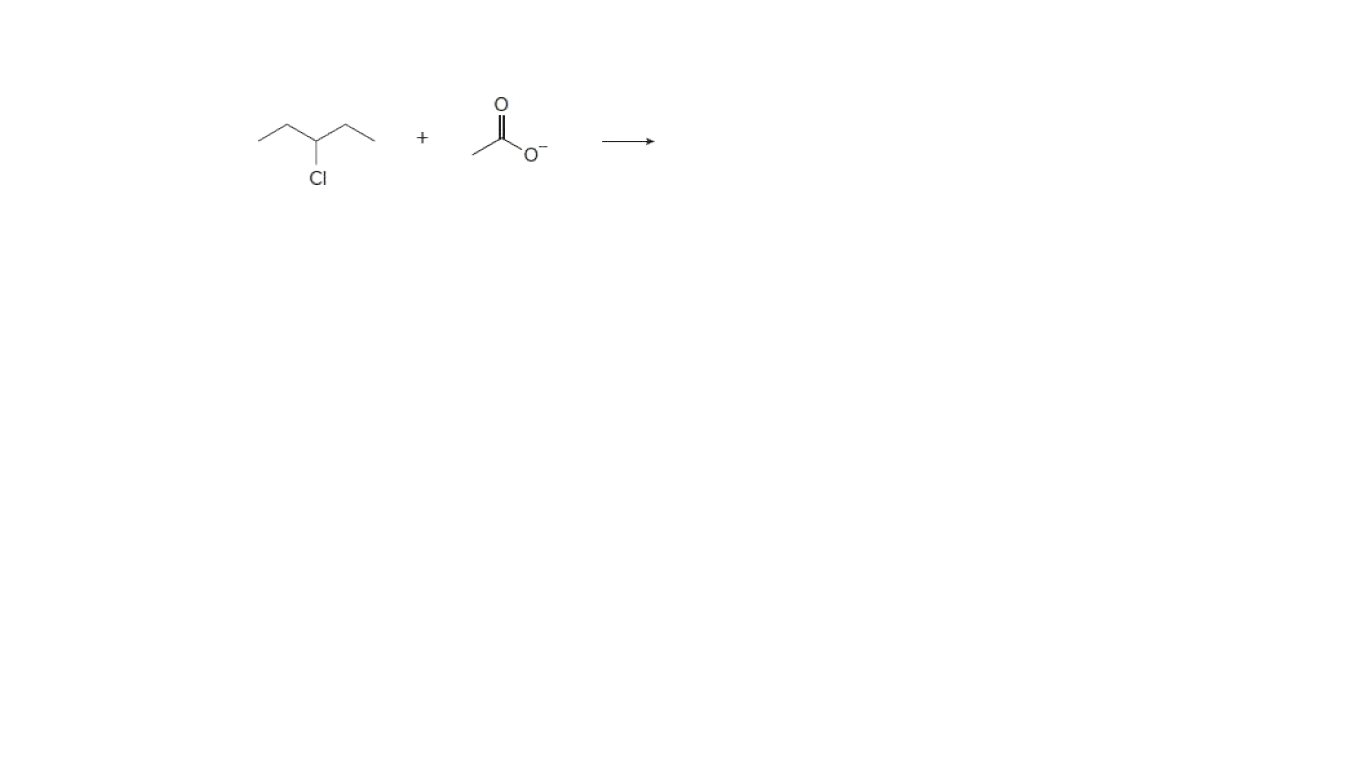
Transcribed Image Text:CI
Expert Solution
Step 1
In nucleophilic substitution reaction the leaving group is replaced by the electron rich species (nucleophile) to form the product. Due to the more electronegativity of the chlorine atom the carbon atom attached to the chlorine is deficient atom. So the the anion i.e. acetate ion ( has electron density) attach at that carbon atom and the reaction take place is the nucleophilic substitution reaction.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

