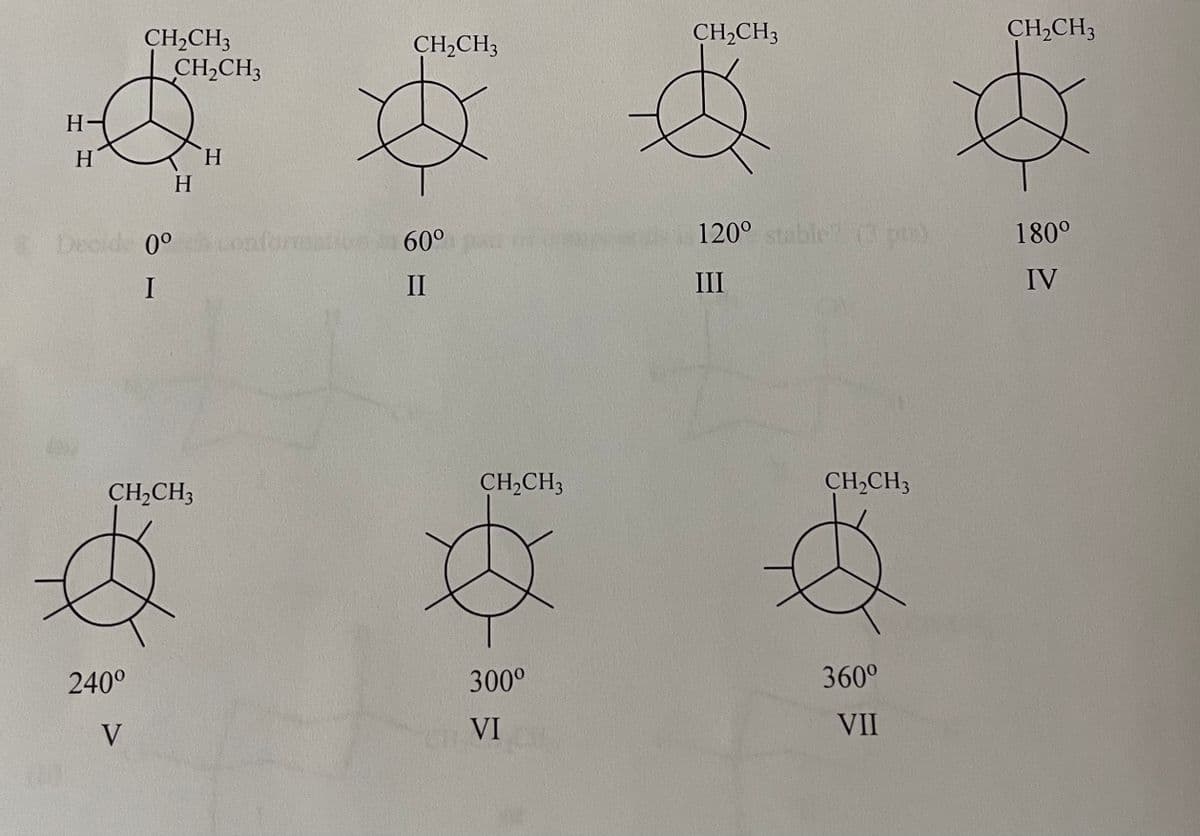
Citing down the C3-C4 bond, and using the drawings below, complete the Newman projections for all staggered and eclipsed conformations of hexane. Which staggered conformation has the lowest energy? Which eclipsed conformation has the highest energy?
Citing down the C3-C4 bond, and using the drawings below, complete the Newman projections for all staggered and eclipsed conformations of hexane. Which staggered conformation has the lowest energy? Which eclipsed conformation has the highest energy?
Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.3: Stability Of Cycloalkanes: Ring Strain
Problem 8P: Each H↔H eclipsing interaction in ethane costs about 4.0 kJ/mol. How many such interactions are...
Related questions
Question
Citing down the C3-C4 bond, and using the drawings below, complete the Newman projections for all staggered and eclipsed conformations of hexane. Which staggered conformation has the lowest energy? Which eclipsed conformation has the highest energy?
Transcribed Image Text:CH2CH3
CH,CH3
CH,CH3
CH,CH3
CH,CH3
H•
H.
H.
H
3
Decide 0° onforest
60°
120°ble (3 p)
180°
I
II
III
IV
CH,CH3
CH,CH3
CH,CH3
240°
300°
360°
V.
VI
VII
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co