Classify each of the possible reactions according to whether a precipitate will form or will not form. KOH(aq) + FeCl3(aq) - Precipitate forms →? LINO3(aq) + Na₂SO4 (aq) → ? Na₂CO3(aq) + CuCl₂ (aq) - Answer Bank →? KI(aq) + NaCl(aq) →? CrCl,(aq) + Li,CO, (aq) - No reaction → ?
Classify each of the possible reactions according to whether a precipitate will form or will not form. KOH(aq) + FeCl3(aq) - Precipitate forms →? LINO3(aq) + Na₂SO4 (aq) → ? Na₂CO3(aq) + CuCl₂ (aq) - Answer Bank →? KI(aq) + NaCl(aq) →? CrCl,(aq) + Li,CO, (aq) - No reaction → ?
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 45E: When the following solutions are mixed together, what precipitate (if any) will form? a. FeSO4(aq) +...
Related questions
Question
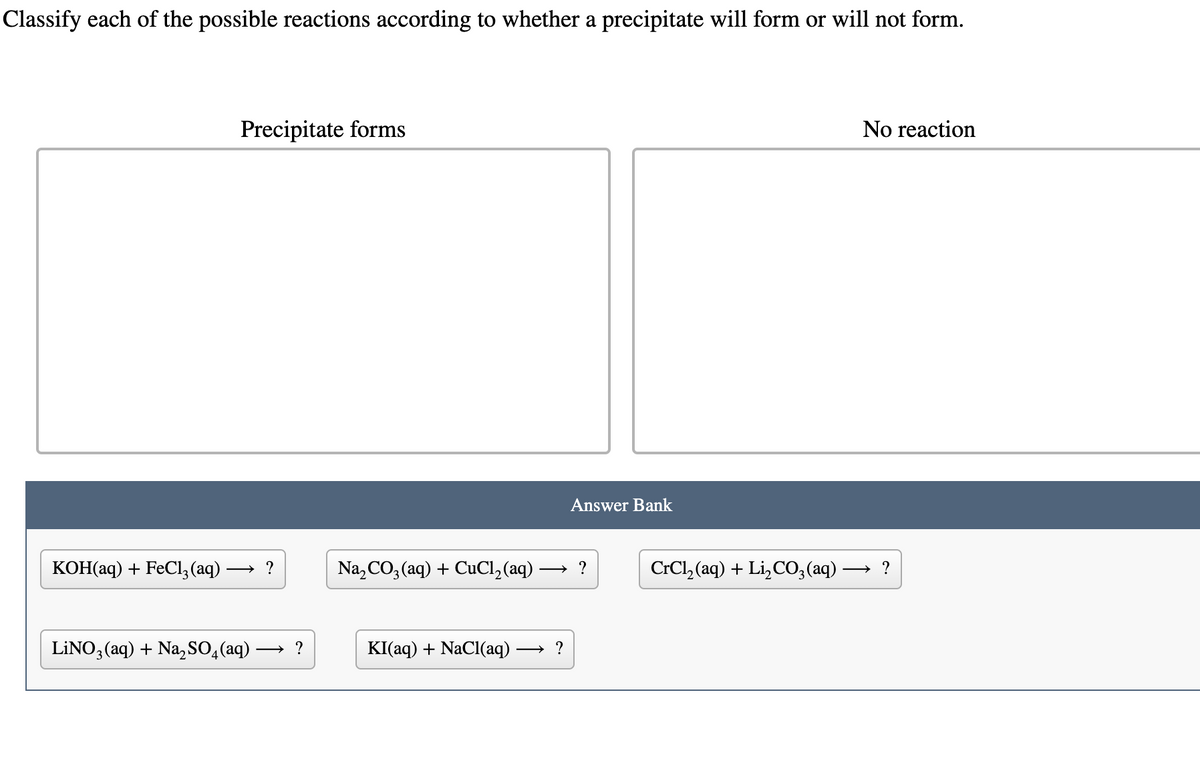
Transcribed Image Text:Classify each of the possible reactions according to whether a precipitate will form or will not form.
KOH(aq) + FeCl3(aq) -
Precipitate forms
→?
LINO3(aq) + Na₂SO4 (aq)
→ ?
Na₂CO3(aq) + CuCl₂ (aq) -
Answer Bank
→?
KI(aq) + NaCl(aq) →?
CrCl,(aq) + Li,CO, (aq) -
No reaction
→ ?
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning