Classify the following by the sign of AE for the system. Drag the appropriate items to their respective bins. If no definitive classification can be made, drag the item into the bin labeled "Not enough data." Reset Help The system contracts and the surroundings get colder. The system expands and the surroundings get colder. The system expands and the surroundings get hotter. The system contracts and the surroundings get hotter. Positive Not enough data Negative
Classify the following by the sign of AE for the system. Drag the appropriate items to their respective bins. If no definitive classification can be made, drag the item into the bin labeled "Not enough data." Reset Help The system contracts and the surroundings get colder. The system expands and the surroundings get colder. The system expands and the surroundings get hotter. The system contracts and the surroundings get hotter. Positive Not enough data Negative
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 9P
Related questions
Question
Pls help ASAP. Pls give proper units and significant digits to three. Pls circle the final answer as well.
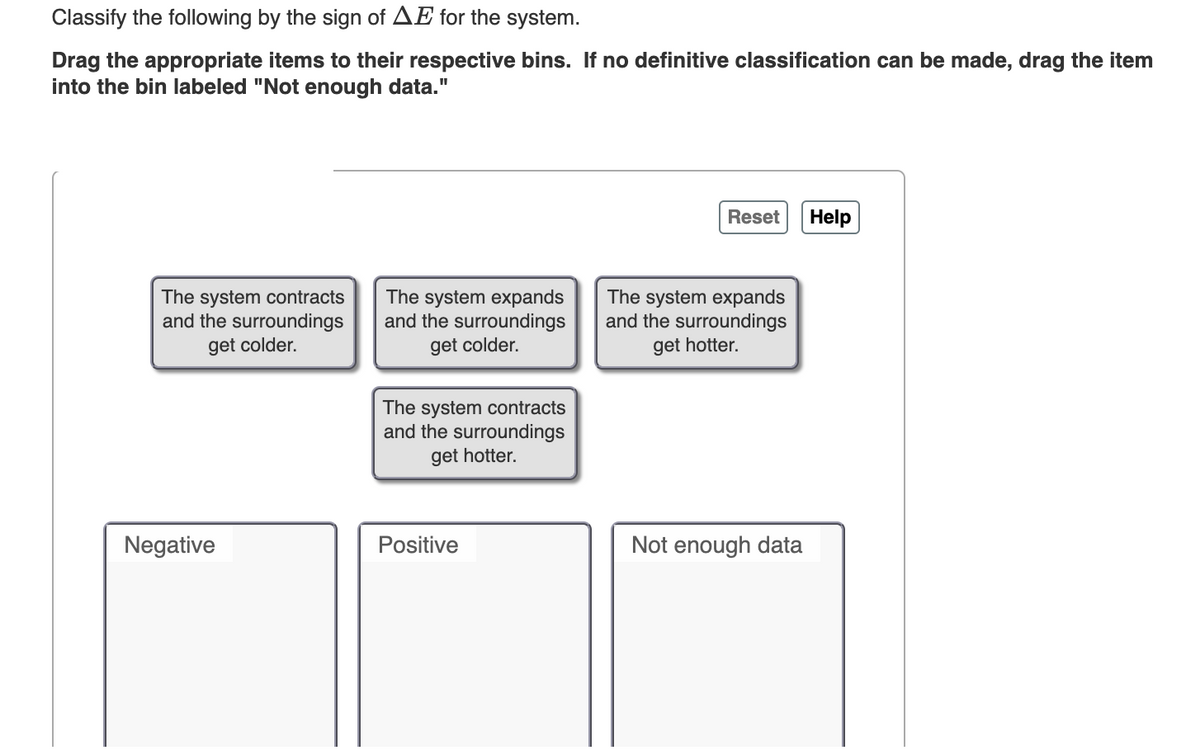
Transcribed Image Text:Classify the following by the sign of AE for the system.
Drag the appropriate items to their respective bins. If no definitive classification can be made, drag the item
into the bin labeled "Not enough data."
Reset Help
The system contracts
and the surroundings
get colder.
The system expands
and the surroundings
get colder.
The system expands
and the surroundings
get hotter.
The system contracts
and the surroundings
get hotter.
Positive
Not enough data
Negative
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning