Classify the following reactions as precipitation reactions, oxidation-reduction reactions, or acid-base reactions. Drag each item to the appropriate bin. • View Available Hint(s) Reset Help Sr(CH3COO)2(aq)+ (NH4)2SO4(aq) –→ SESO4(s)+ 2NH,CH,COO(aq) Cu(CH3COO)2(aq)+ (NH4)2C2O4(aq) → CuC2Ò4(s) + 2NH,CH;COO(aq) 4Al(s) + 302(g) → 2A1½O3(s) | 2Hg(1) + O2(g) → 2HºO(s) Mg(s) + Cu?- (aq) → Mg2+ (aq) + Cu(s) Mg(OH)2(s) + 2HC1(aq) → 2H20(1) + MgCl2 (aq) NaOH(aq) + HCi(aq) → Načl(aq) + H20(1) Precipitation Oxidation-reduction Acid-base
Classify the following reactions as precipitation reactions, oxidation-reduction reactions, or acid-base reactions. Drag each item to the appropriate bin. • View Available Hint(s) Reset Help Sr(CH3COO)2(aq)+ (NH4)2SO4(aq) –→ SESO4(s)+ 2NH,CH,COO(aq) Cu(CH3COO)2(aq)+ (NH4)2C2O4(aq) → CuC2Ò4(s) + 2NH,CH;COO(aq) 4Al(s) + 302(g) → 2A1½O3(s) | 2Hg(1) + O2(g) → 2HºO(s) Mg(s) + Cu?- (aq) → Mg2+ (aq) + Cu(s) Mg(OH)2(s) + 2HC1(aq) → 2H20(1) + MgCl2 (aq) NaOH(aq) + HCi(aq) → Načl(aq) + H20(1) Precipitation Oxidation-reduction Acid-base
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 6CR: xplain to your friend what chemists mean by a precipitation reaction. What is the driving force in a...
Related questions
Question
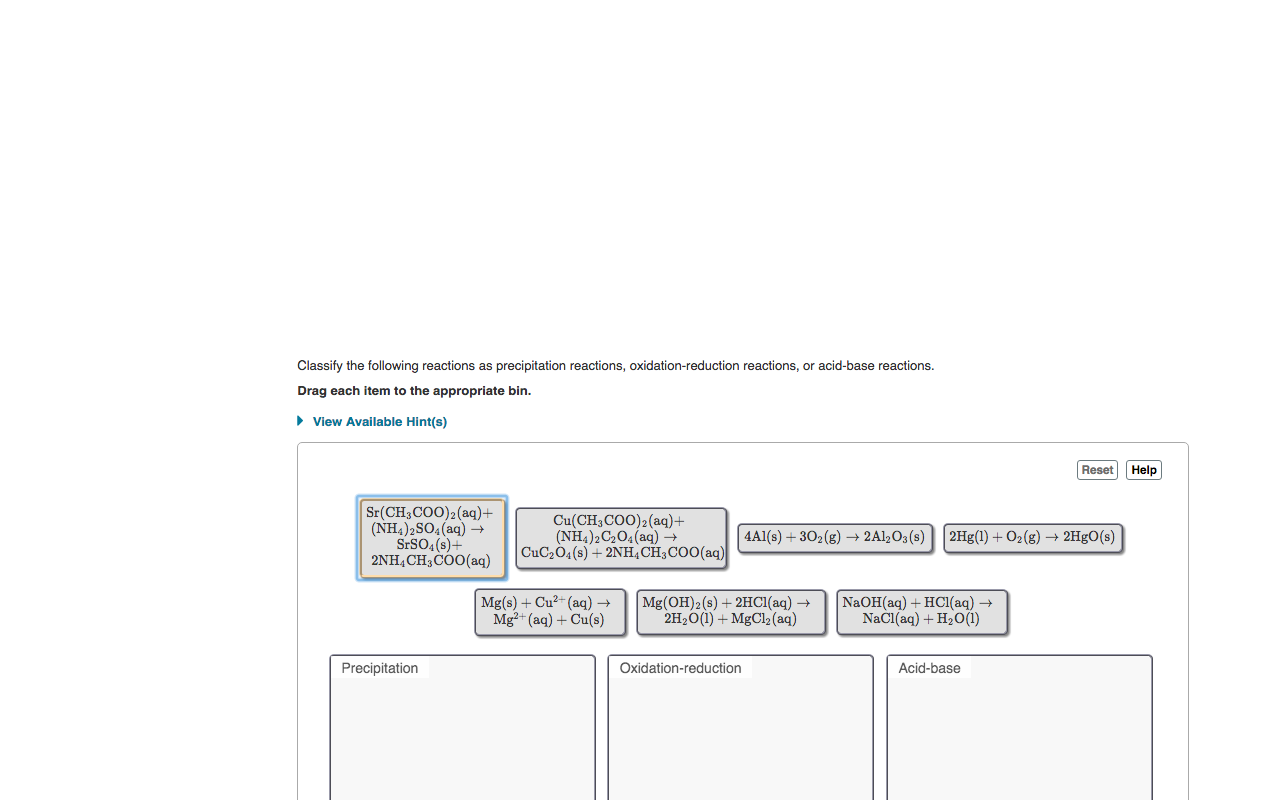
Transcribed Image Text:Classify the following reactions as precipitation reactions, oxidation-reduction reactions, or acid-base reactions.
Drag each item to the appropriate bin.
• View Available Hint(s)
Reset
Help
Sr(CH3COO)2(aq)+
(NH4)2SO4(aq) –→
SESO4(s)+
2NH,CH,COO(aq)
Cu(CH3COO)2(aq)+
(NH4)2C2O4(aq) →
CuC2Ò4(s) + 2NH,CH;COO(aq)
4Al(s) + 302(g) → 2A1½O3(s)
| 2Hg(1) + O2(g) → 2HºO(s)
Mg(s) + Cu?- (aq) →
Mg2+ (aq) + Cu(s)
Mg(OH)2(s) + 2HC1(aq) →
2H20(1) + MgCl2 (aq)
NaOH(aq) + HCi(aq) →
Načl(aq) + H20(1)
Precipitation
Oxidation-reduction
Acid-base
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning