Classify the statements about redox reactions as true or false. True False Answer Bank If there are no changes in the oxidation state of the reactants or products of a particular reaction, that reaction is not a redox reaction. A reducing agent gets oxidized as it reacts. If something is reduced, it is formally losing electrons. If something is oxidized, it is formally losing electrons. In the redox reaction Fe3+ + Co² + Fe2 + + Co³ +, Fe3 + is the reducing agent and Co+ is the oxidizing agent. > Oxidizing agents can convert CO into CO,.
Classify the statements about redox reactions as true or false. True False Answer Bank If there are no changes in the oxidation state of the reactants or products of a particular reaction, that reaction is not a redox reaction. A reducing agent gets oxidized as it reacts. If something is reduced, it is formally losing electrons. If something is oxidized, it is formally losing electrons. In the redox reaction Fe3+ + Co² + Fe2 + + Co³ +, Fe3 + is the reducing agent and Co+ is the oxidizing agent. > Oxidizing agents can convert CO into CO,.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 113QRT
Related questions
Question
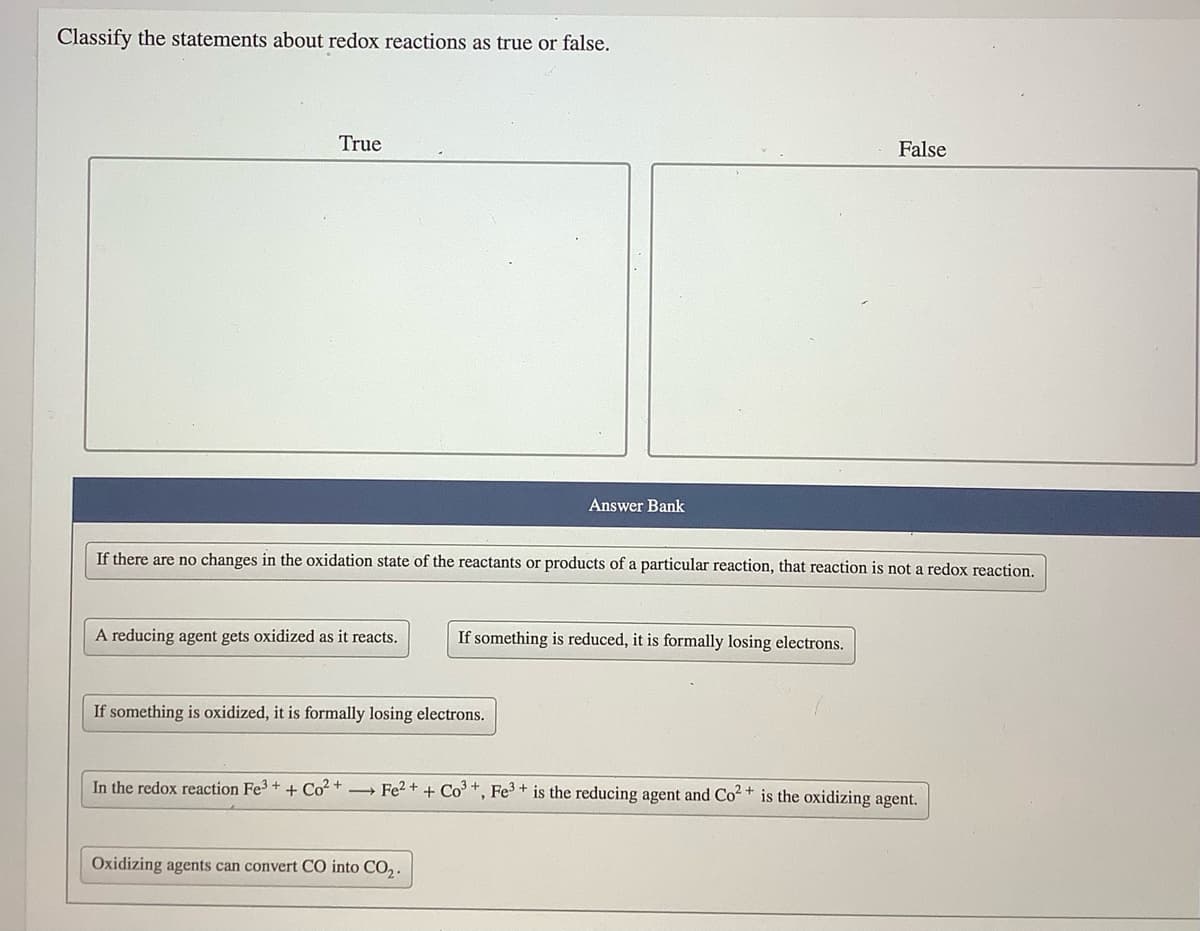
Transcribed Image Text:Classify the statements about redox reactions as true or false.
True
False
Answer Bank
If there are no changes in the oxidation state of the reactants or products of a particular reaction, that reaction is not a redox reaction.
A reducing agent gets oxidized as it reacts.
If something is reduced, it is formally losing electrons.
If something is oxidized, it is formally losing electrons.
In the redox reaction Fe3 +
+ Co? +
→ Fe2 + + Co +, Fe3 + is the reducing agent and Co2+ is the oxidizing agent.
Oxidizing agents can convert CO into CO,.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning