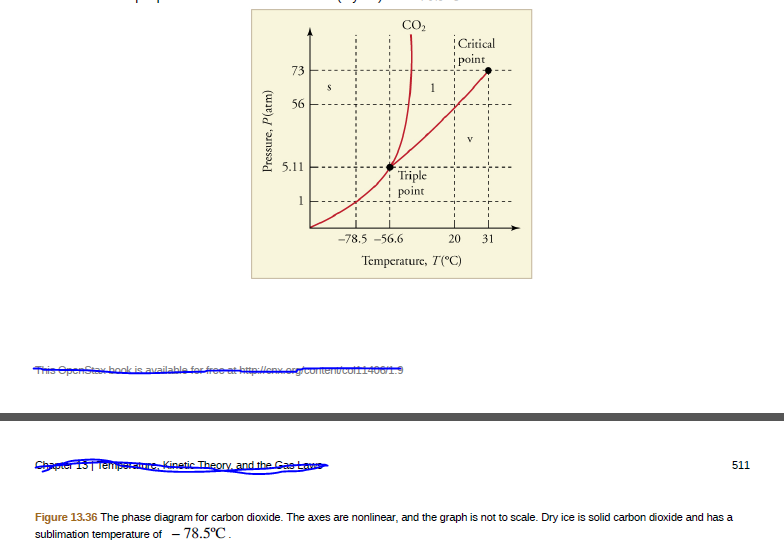
CO, Critical point 73 56 5.11 Triple point -78.5 -56.6 20 31 Temperature, T(°C) Tris epenstabook is available fo tpllen.ougcomenƯCo14001 Chaprer STTer sraue Kinetic Theory, and the Gasto 511 Figure 13.36 The phase diagram for carbon dioxide. The axes are nonlinear, and the graph is not to scale. Dry ice is solid carbon dioxide and has a sublimation temperature of - 78.5°C. Pressure, P(atm)
CO, Critical point 73 56 5.11 Triple point -78.5 -56.6 20 31 Temperature, T(°C) Tris epenstabook is available fo tpllen.ougcomenƯCo14001 Chaprer STTer sraue Kinetic Theory, and the Gasto 511 Figure 13.36 The phase diagram for carbon dioxide. The axes are nonlinear, and the graph is not to scale. Dry ice is solid carbon dioxide and has a sublimation temperature of - 78.5°C. Pressure, P(atm)
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 39P: Typical molecular speeds (vrms) are large, even at low temperatures. What is vrms for helium atoms...
Related questions
Question
Can carbon dioxide be liquefied at room temperature ( 20ºC )? If so, how? If not, why not?
Transcribed Image Text:CO,
Critical
point
73
56
5.11
Triple
point
-78.5 -56.6
20
31
Temperature, T(°C)
Tris epenstabook is available fo
tpllen.ougcomenƯCo14001
Chaprer STTer
sraue Kinetic Theory, and the Gasto
511
Figure 13.36 The phase diagram for carbon dioxide. The axes are nonlinear, and the graph is not to scale. Dry ice is solid carbon dioxide and has a
sublimation temperature of - 78.5°C.
Pressure, P(atm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning