CO2 + H,0 = H,CO3 = H* + HCO3 = 2H* + CO32- carbonic acid bicarbonate carbonate Shown above are the chemical equilibria of the carbonate buffer system. What happens to this equilibrium when an HCl is added to it? O The pH changes quickly as the equilibrium shifts to the right. O The pH changes quickly as the equilibrium shifts to the left. O The pH changes slowly as the equilibrium shifts to the right.
CO2 + H,0 = H,CO3 = H* + HCO3 = 2H* + CO32- carbonic acid bicarbonate carbonate Shown above are the chemical equilibria of the carbonate buffer system. What happens to this equilibrium when an HCl is added to it? O The pH changes quickly as the equilibrium shifts to the right. O The pH changes quickly as the equilibrium shifts to the left. O The pH changes slowly as the equilibrium shifts to the right.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 102CWP: Consider the following acids and bases: HCO2H Ka = 1.8 104 HOBr Ka = 2.0 109 (C2H5)2NH Kb = 1.3 ...
Related questions
Question
2?
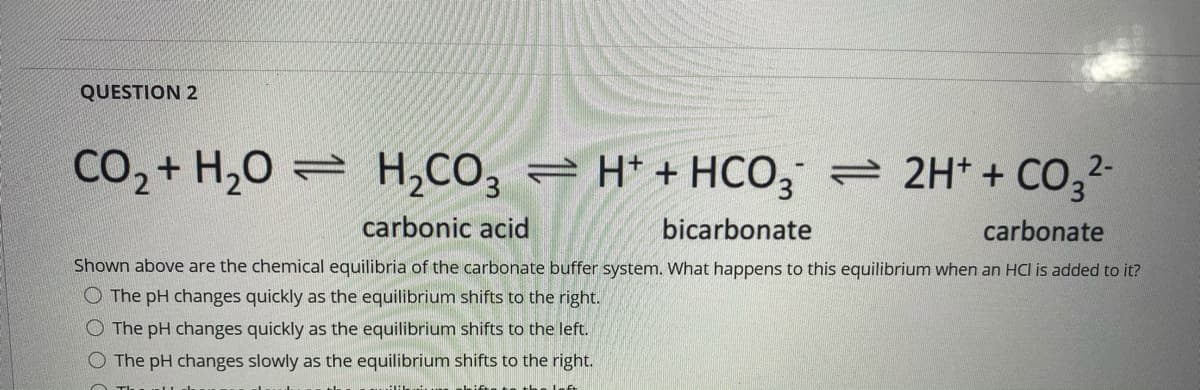
Transcribed Image Text:QUESTION 2
CO2 + H,0 = H,CO, = H* + HCO, = 2H* + CO,2-
carbonic acid
bicarbonate
carbonate
Shown above are the chemical equilibria of the carbonate buffer system. What happens to this equilibrium when an HCI is added to it?
O The pH changes quickly as the equilibrium shifts to the right.
O The pH changes quickly as the equilibrium shifts to the left.
The pH changes slowly as the equilibrium shifts to the right.
Expert Solution
Step 1
CO2 +H2 <=> H2CO3 <=> H+ + HCO3-<=> 2H++CO32-
In this equilibrium condition, HCl is added them initially Hydrogen ions concentration increases, which neutralised by carbonate ions present in it to form again bicarbonate and carbonic acid and Equilibrium shifts towards Reactants side, or left side.
Here Le Chatelier's principle is applicable, according to that any change in Equilibrium condition can neutralised by system automatically after some time to minimize the change.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
