Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.22QAP
Related questions
Question
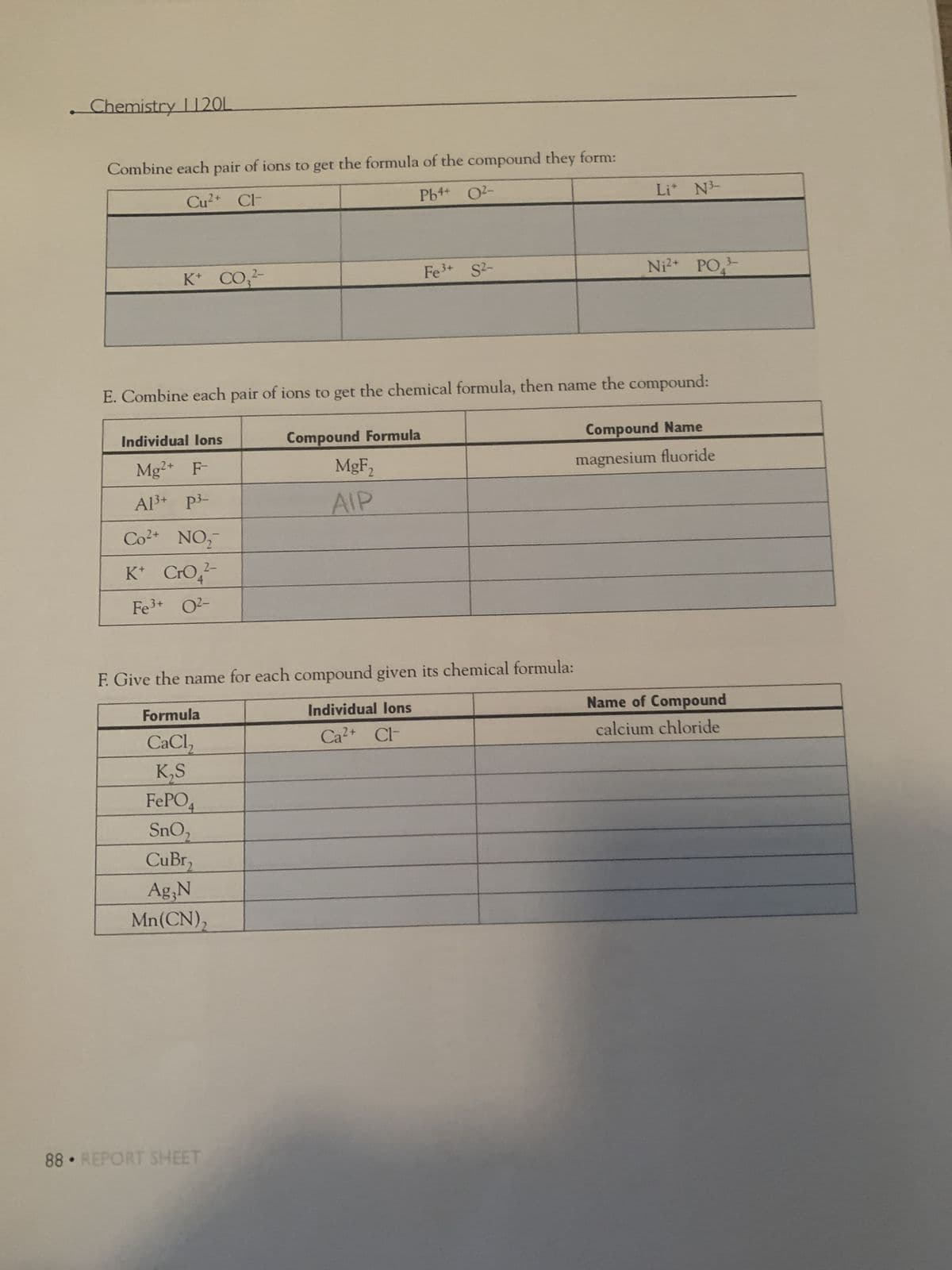
Transcribed Image Text:Chemistry 1120L
Combine each pair of ions to get the formula of the compound they form:
Cu²+ Cl
Pb++ O²-
K+ CO₂2-
E. Combine each pair of ions to get the chemical formula, then name the
Individual lons
Mg2+ F-
Al³+ P³-
Co²+ NO₂
K* CrO4²-
2-
Fe³+ O²-
Ag,N
Mn(CN)₂
Fe³+ S²-
Compound Formula
MgF₂
AIP
88 REPORT SHEET
F. Give the name for each compound given its chemical formula:
Formula
Individual lons
Ca²+ Cl-
CaCl₂
K₂S
FePO4
SnO₂
CuBr₂
Li* N
Ni²+ PO
compound:
Compound Name
magnesium fluoride
Name of Compound
calcium chloride
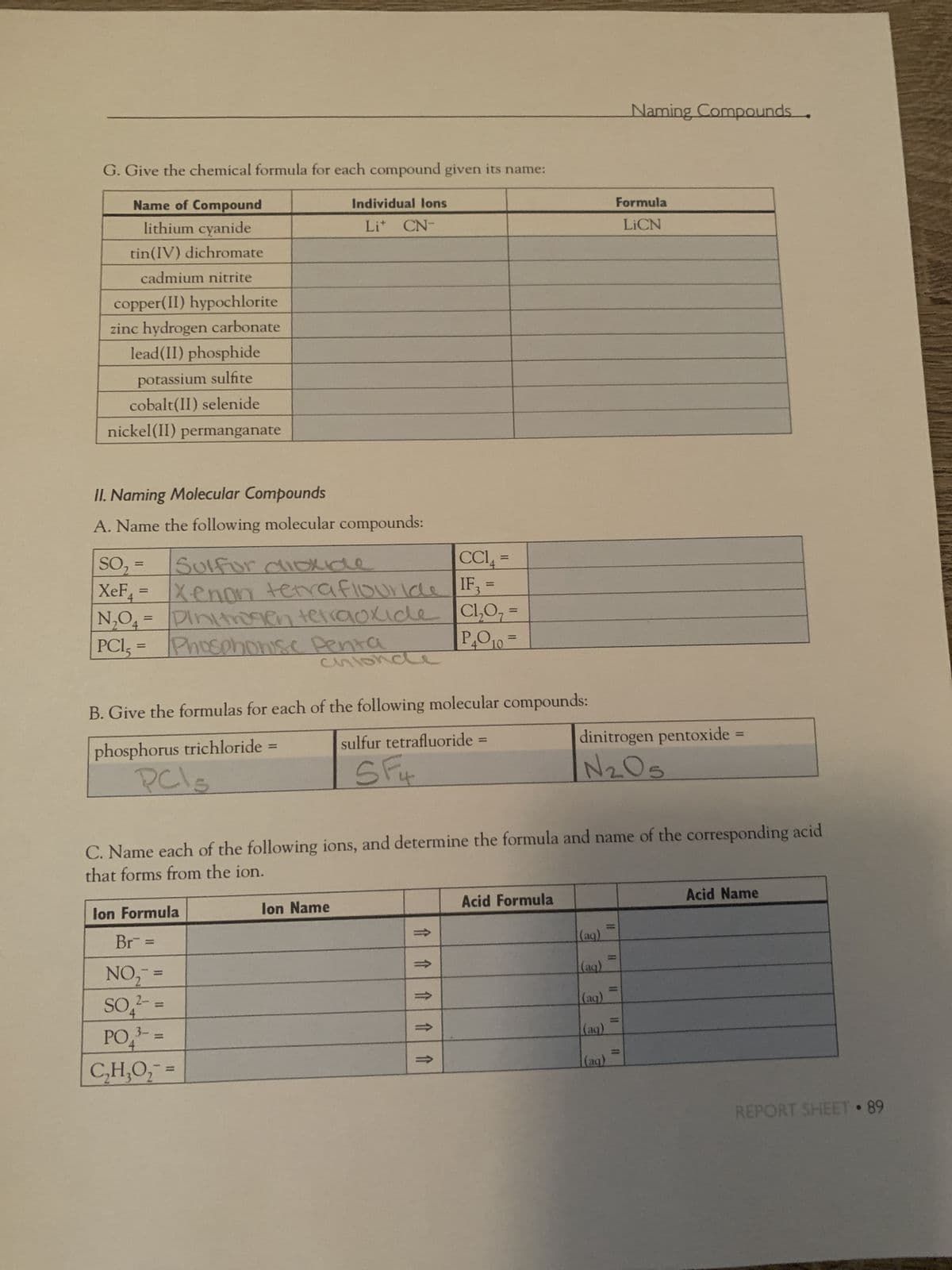
Transcribed Image Text:G. Give the chemical formula for each compound given its name:
Name of Compound
lithium cyanide
tin(IV) dichromate
cadmium nitrite
copper(II) hypochlorite
zinc hydrogen carbonate
lead(II) phosphide
potassium sulfite
cobalt(II) selenide
nickel(II) permanganate
II. Naming Molecular Compounds
A. Name the following molecular compounds:
=
CCIA
Sulfur dioxide
SO₂
IF3
IF3 =
XeF₁ = Xenon terraflouride
N₂O4= Dinitrogen tetraoxide C1₂
Cl₂O₂
P₁0₁0=
PC1, =
Individual lons
Lit CN-
Phosphonise Penta
lon Formula
Br =
NO₂ =
2-
SO4²- =
chlond
B. Give the formulas for each of the following molecular compounds:
phosphorus trichloride
PCIS
3-
PO₁³-=
C₂H₂O₂¯ =
lon Name
sulfur tetrafluoride
SF4
=
=
1 1 1 1 1
C. Name each of the following ions, and determine the formula and name of the corresponding acid
that forms from the ion.
Acid Formula
dinitrogen pentoxide
N ₂05
(aq)
(aq)
[(ag)
(aq)
Naming Compounds.
(aq)
Formula
LICN
||
Acid Name
REPORT SHEET • 89
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

