Combustion of hydrocarbons such as pentane (C5H₁2) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. x10 X 5 ? 2. Suppose 0.460 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 significant digits. Explanation Check Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A ES
Combustion of hydrocarbons such as pentane (C5H₁2) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. x10 X 5 ? 2. Suppose 0.460 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 significant digits. Explanation Check Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A ES
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section: Chapter Questions
Problem 84QRT: Oxygen is not normally found in positive oxidation states, but when it is combined with fluorine in...
Related questions
Question
Please answer all the parts of the following question
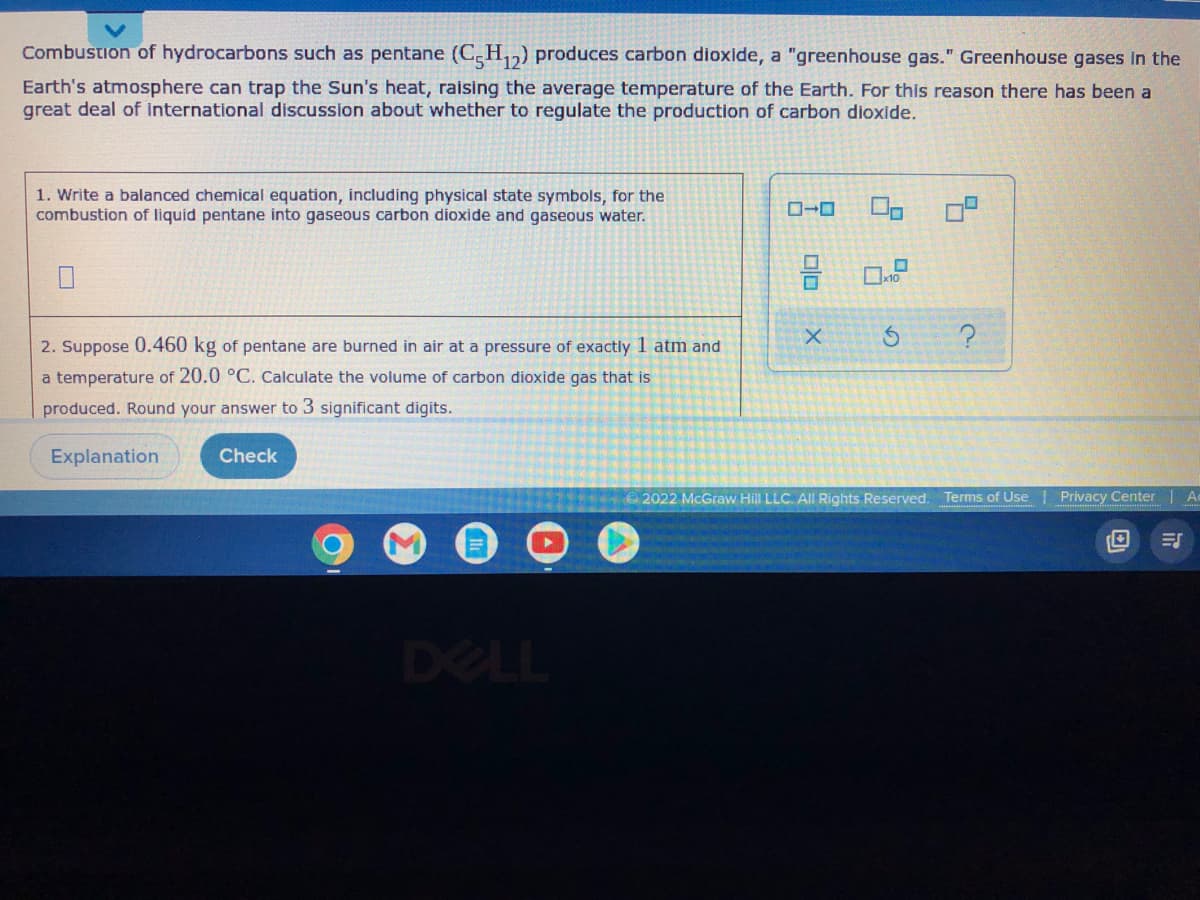
Transcribed Image Text:Combustion of hydrocarbons such as pentane (C5H₁2) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the
Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a
great deal of international discussion about whether to regulate the production of carbon dioxide.
1. Write a balanced chemical equation, including physical state symbols, for the
combustion of liquid pentane into gaseous carbon dioxide and gaseous water.
x10
X
5
2. Suppose 0.460 kg of pentane are burned in air at a pressure of exactly 1 atm and
?
a temperature of 20.0 °C. Calculate the volume of carbon dioxide gas that is
produced. Round your answer to 3 significant digits.
Explanation
Check
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A
DELL
T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning