Compare the densities of the objects in question 1 and 2 to the densities of materials found in Table 3.1. What materials were used to construct these objects? Table 3.1: Densities of Some Solid Materials Material Density (g/cm) Density (g/cm) Material Copper 8.9 Nylon 1.13 Brass 8.0 ABS polymer 1.0 - 1.1 High density polyethylene polymer Low Density Polyethylene polymer Iron 7.9 0.94 Steel 7.6 0.91 Aluminum 2.7 Oak wood 0.6-0.9 PVC 1.40 Pine wood 0.48-0.56 Acrylic 1.18 Balsa wood 0.16
Compare the densities of the objects in question 1 and 2 to the densities of materials found in Table 3.1. What materials were used to construct these objects? Table 3.1: Densities of Some Solid Materials Material Density (g/cm) Density (g/cm) Material Copper 8.9 Nylon 1.13 Brass 8.0 ABS polymer 1.0 - 1.1 High density polyethylene polymer Low Density Polyethylene polymer Iron 7.9 0.94 Steel 7.6 0.91 Aluminum 2.7 Oak wood 0.6-0.9 PVC 1.40 Pine wood 0.48-0.56 Acrylic 1.18 Balsa wood 0.16
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Having a problem with question 3. It refers to question 1 &2 to answer number 3
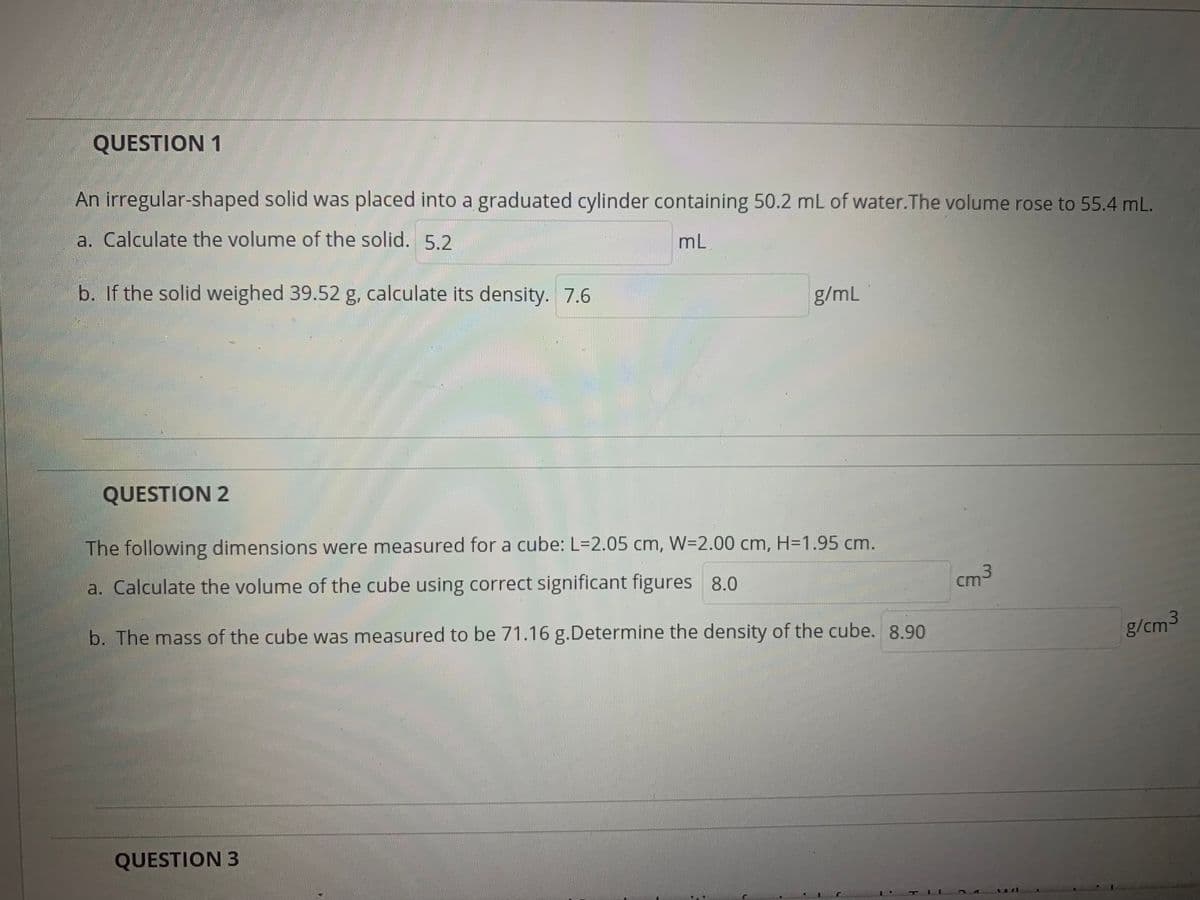
Transcribed Image Text:QUESTION 1
An irregular-shaped solid was placed into a graduated cylinder containing 50.2 mL of water.The volume rose to 55.4 mL.
a. Calculate the volume of the solid. 5.2
mL
b. If the solid weighed 39.52 g, calculate its density. 7.6
g/mL
QUESTION 2
The following dimensions were measured for a cube: L=2.05 cm, W=2.00 cm, H=1.95 cm.
a. Calculate the volume of the cube using correct significant figures 8.0
cm
b. The mass of the cube was measured to be 71.16 g.Determine the density of the cube. 8.90
g/cm3
QUESTION 3
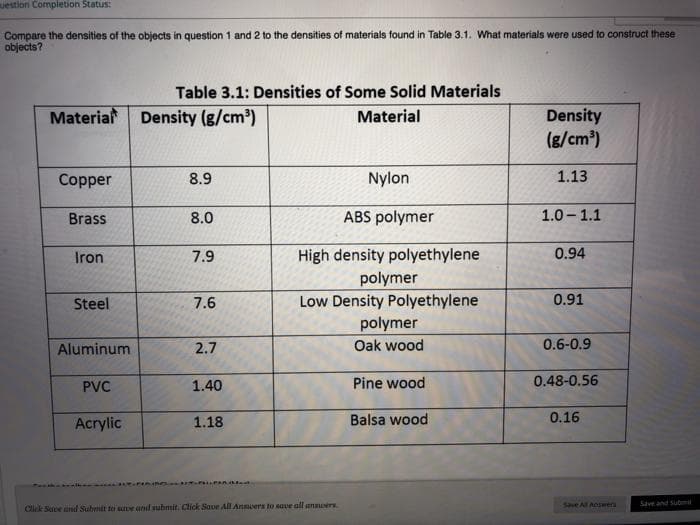
Transcribed Image Text:uestion Completion Status:
Compare the densities of the objects in question 1 and 2 to the densities of materials found in Table 3.1. What materials were used to construct these
objects?
Table 3.1: Densities of Some Solid Materials
Material
Density (g/cm)
Density
(g/cm)
Material
Copper
8.9
Nylon
1.13
Brass
8.0
ABS polymer
1.0 - 1.1
Iron
7.9
High density polyethylene
0.94
polymer
Steel
7.6
Low Density Polyethylene
0.91
polymer
Aluminum
2.7
Oak wood
0.6-0.9
PVC
1.40
Pine wood
0.48-0.56
Acrylic
1.18
Balsa wood
0.16
Save A Aoswera
Save and Submii
Click Suce and Submit to ue and submit. Click Save All Ansuers to save all anaIers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY