Compare the energy (in joules) carried by an X-ray pho- ton (wavelength A = 0.20 nm) with that carried by an AM radio wave photon (A = 200 m). Calculate the 1.00 mol of each type of photon. What effect do you expect each type of radiation to have for inducing chemi- cal reactions in the substances through which it passes? energy of
Compare the energy (in joules) carried by an X-ray pho- ton (wavelength A = 0.20 nm) with that carried by an AM radio wave photon (A = 200 m). Calculate the 1.00 mol of each type of photon. What effect do you expect each type of radiation to have for inducing chemi- cal reactions in the substances through which it passes? energy of
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 48AP: Compare the energy (in joules) carried by an X-ray photon (wavelength=0.20nm) with that carried by...
Related questions
Question
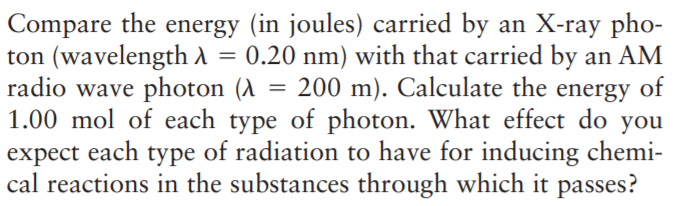
Transcribed Image Text:Compare the energy (in joules) carried by an X-ray pho-
ton (wavelength A = 0.20 nm) with that carried by an AM
radio wave photon (A = 200 m). Calculate the
1.00 mol of each type of photon. What effect do you
expect each type of radiation to have for inducing chemi-
cal reactions in the substances through which it passes?
energy
of
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning