Compare this value with a standard value. Compute the percent difference between the two and discuss errors and source of errors. Metals Steel Copper Aluminum (x 10-6/°C) (x 10-6/°C) (x 10-6/°C) Observed 12.7 17.3 24.2 Value Standard Value Formula: Percentage Difference = x 100% Where: Steel: | V₁ = Observed value V, Standard value = 12.7 - V₂ PD = (12.7 + ₂)/2 17.3 -16.5 (17.3 + 16.5)/2 PD = 4.7% 24.2 - 23.6 (24.2 + 23.6)/2 PD = 2.5% Copper: PD Aluminum: Vo - Vs (Vo+V₂)/2 PD = x 100% x 100% x 100%
Compare this value with a standard value. Compute the percent difference between the two and discuss errors and source of errors. Metals Steel Copper Aluminum (x 10-6/°C) (x 10-6/°C) (x 10-6/°C) Observed 12.7 17.3 24.2 Value Standard Value Formula: Percentage Difference = x 100% Where: Steel: | V₁ = Observed value V, Standard value = 12.7 - V₂ PD = (12.7 + ₂)/2 17.3 -16.5 (17.3 + 16.5)/2 PD = 4.7% 24.2 - 23.6 (24.2 + 23.6)/2 PD = 2.5% Copper: PD Aluminum: Vo - Vs (Vo+V₂)/2 PD = x 100% x 100% x 100%
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 2.1CYU: Check Your Understanding The recommended daily amount of vitamin B3 or niacin, C6NH5 O2, for women...
Related questions
Question
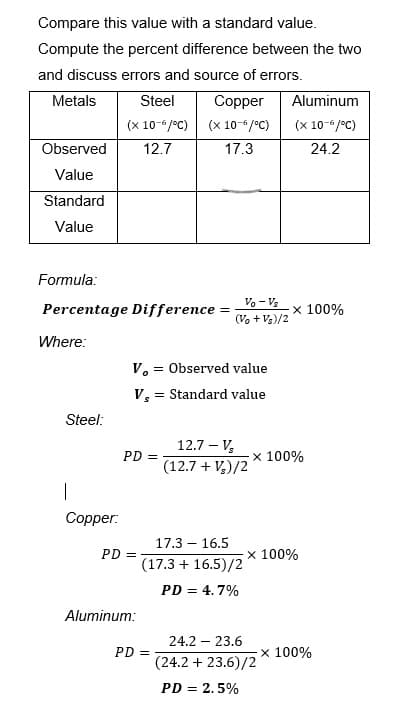
Transcribed Image Text:Compare this value with a standard value.
Compute the percent difference between the two
and discuss errors and source of errors.
Metals
Steel
Copper
Aluminum
(x 10-6/°C)
(x 10-6/°C)
(x 10-6/°C)
Observed
12.7
17.3
24.2
Value
Standard
Value
Formula:
Percentage Difference =
x 100%
Where:
Steel:
|
Copper:
V. Observed value
=
Vs=
= Standard value
12.7 - V₂
PD =
(12.7 + V₂)/2
17.3 - 16.5
(17.3 + 16.5)/2
PD = 4.7%
24.2 - 23.6
(24.2 + 23.6)/2
PD = 2.5%
PD =
Aluminum:
Vo - Vs
(Vo + Vs)/2
PD =
x 100%
x 100%
x 100%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning