Chapter14: Acids And Bases
Section: Chapter Questions
Problem 129E: Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral,...
Related questions
Question
100%
Can you please help me complete the first one and double check my answer on the second to see if I got it correct. Thank you
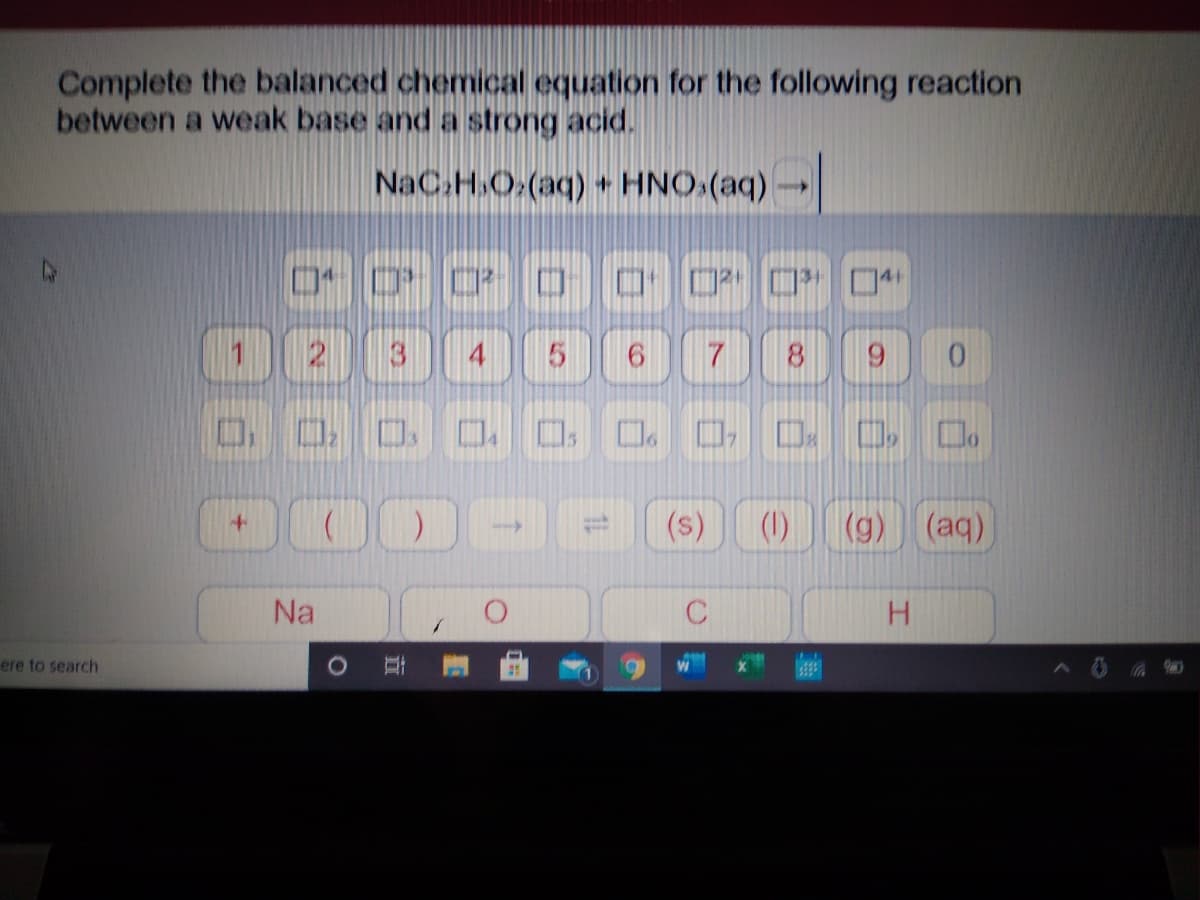
Transcribed Image Text:Complete the balanced chemical equation for the following reaction
between a weak base and a strong acid.
NaC.H.O.(aq) + HNO.(aq)-
口 □* □4
000
8.
9.
D D. D O 0. 0. 0 D. D 0.
(s)
(1)
(g) (aq)
Na
H.
ere to search
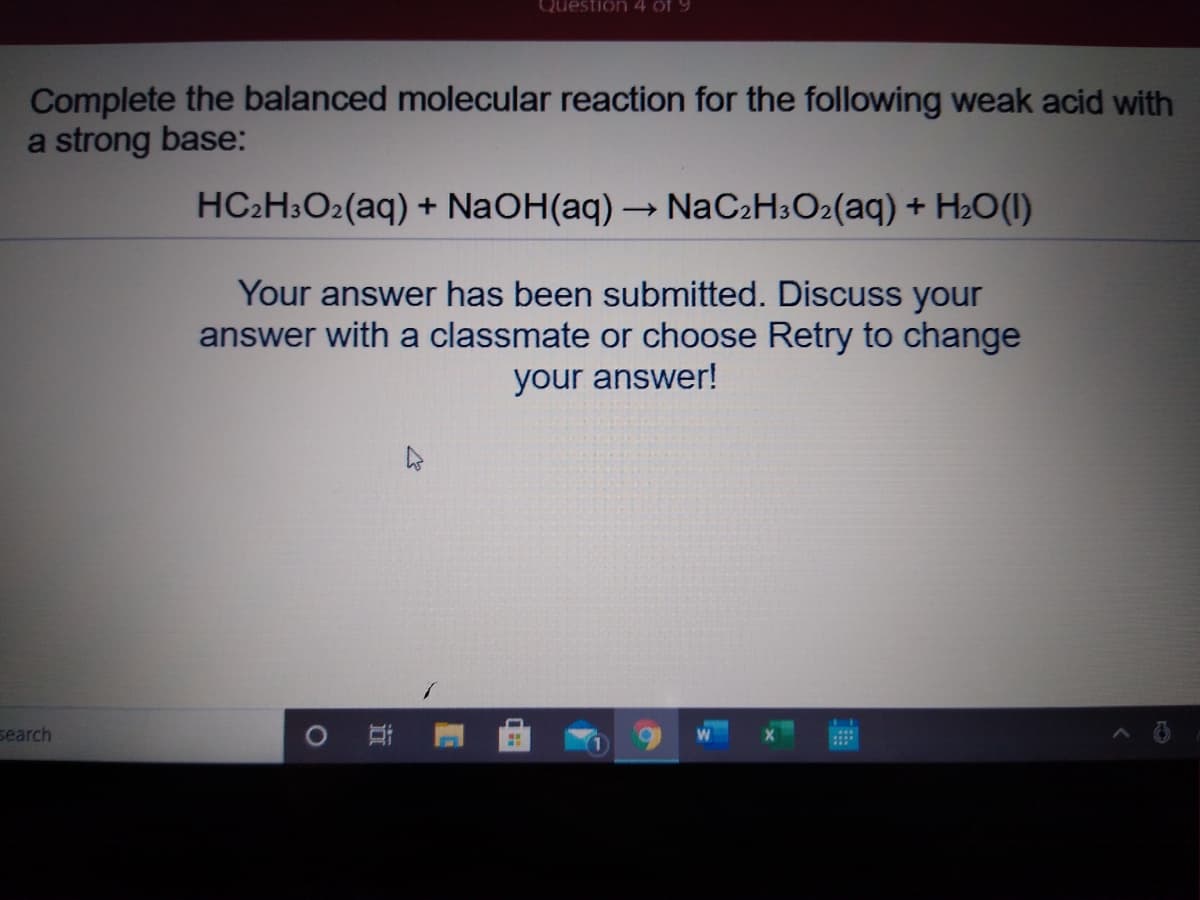
Transcribed Image Text:Question 4 of 9
Complete the balanced molecular reaction for the following weak acid with
a strong base:
HC2H3O2(aq) + NaOH(aq) → NaC2H3O2(aq) + H2O(1)
Your answer has been submitted. Discuss your
answer with a classmate or choose Retry to change
your answer!
search
Expert Solution
Step 1
NaC2H3O2 is a weak base, and its name is sodium acetate.
HNO3 is a strong acid and its name is Nitric Acid.
A reaction between An Acid and A base is called a neutralization Reaction.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning