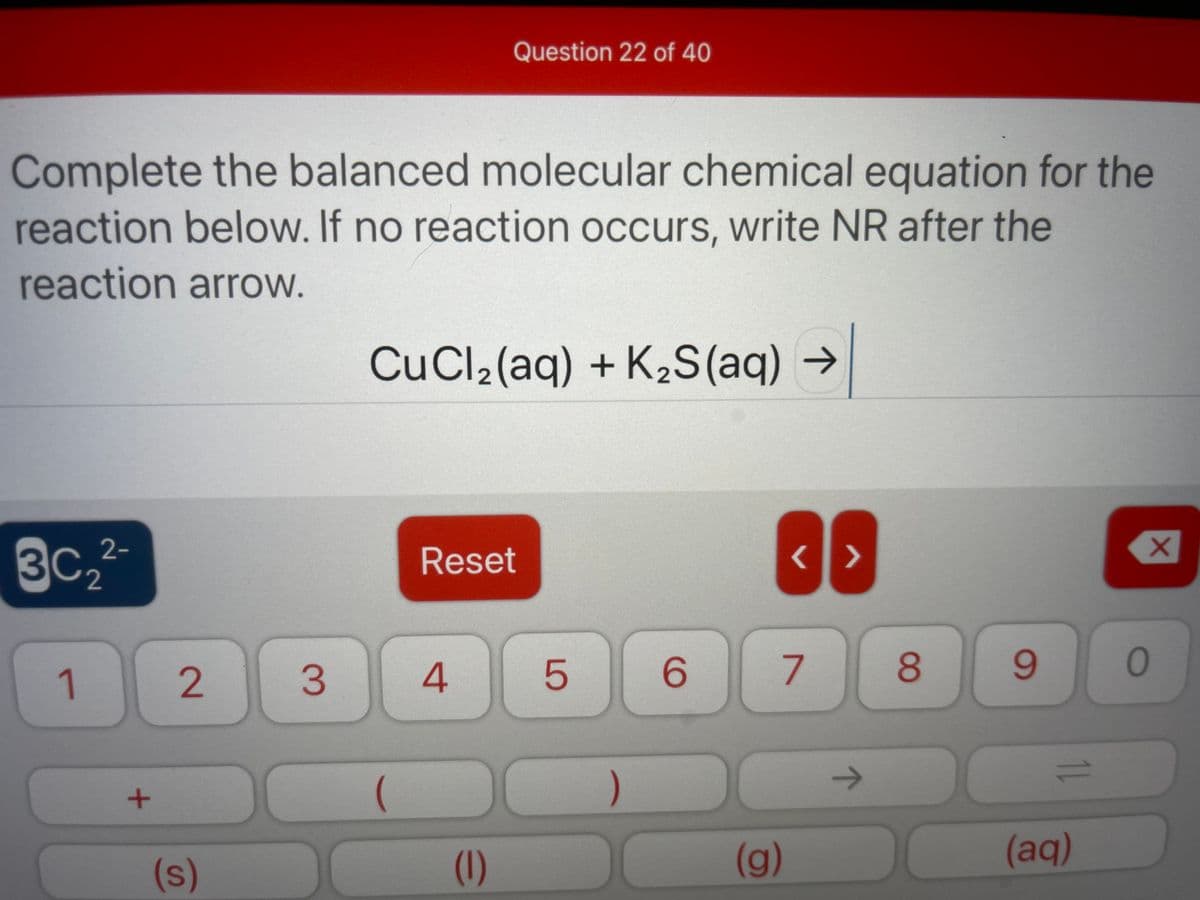
Complete the balanced molecular chemical equation for the eaction below. If no reaction occurs, write NR after the reaction arrow. CuCl2(aq) + K2S(aq) →
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: We know that, Balanced equation means number of atom in reactant side is equal to number of atom in…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Here reaction will occur between chromium (III) sulfate and ammonium carbonate yielding a…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Double displacement reactions are the types of reactions in which the exchange of ions take place…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: The reactants given are Na2CO3 and CsC2H3O2 (aq).
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Given reaction is, CsCl(aq) + K3PO4 (aq) ----->???
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Given K2SO4 (aq) + FeCl3 (aq) → we need to tell whether the reaction takes place and if yes than…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: There are two charges of ions of copper- Copper (I) ions have a 1+ charge. This happens when…
Q: What is the balanced chemical equation that represents the following reaction? goo go O 6H + 2N –-…
A: The answer to the following question is-
Q: Cl2O7(g) + Ca(OH)2(aq)à Ca(ClO4)2(aq) + H2O(l) how do i balance this equation
A: The given chemical reaction is as follows: Cl2O7g + CaOH2aq → CaClO42aq + H2Ol
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Balanced molecular equation is given by,
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: A chemical equation represents the reactants and products participating in a chemical reaction.
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Balanced reaction: A reaction is said to be balanced if all the atoms in reactant and products are…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: The given reaction is a double-displacement reaction, which is defined as a type of chemical…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A:
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A:
Q: (IIIwith carbon to give iron and carbon monde Fe 2 O 2 (s)+3C(s) 2Fe(s)+3CO(g) How many grams of…
A: Mole is the amount of the substance that contains the same number of particles or atoms or…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Given reaction is, Na2CO3(aq)+CsC2H3O2(aq)= ..???
Q: HC1 (aq) + NaOH (aq) – _Zn (s) + CuSO, (aq) - CuSO4 (aq) + _K,CrO4 (aq) –
A: Since you have posted question with multiple sub-parts, we are entitled to answer the first 3 only.…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A:
Q: Complete and balance each combustion reaction equation: a. CH(g) + O2(g) c. CS:(s) + Oz(8) b. C(s) +…
A: Since you have posted multiple sub-parts, the answer for first three sub-parts are given below.…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Complete the balanced molecular chemical equation for the reaction below. If now reaction occurs,…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A:
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Explanation Double Replacement Reactions: If you mix two chemicals, their particles may combine and…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Since the sum of individual atoms on the left side of the equation matches the sum of the same atoms…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: A balanced equation is the equation in which equal number of atoms are present on both the sides of…
Q: How many grams of CO₂ can you produce by reacting 10.85 g of Fe₂O₃ with excess CO according to the…
A:
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: To write the product for the below equation Ba(ClO4)2(aq)+RbOH(aq)→
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Balanced chemical equation of a reaction is written according to law of conservation of mass.…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Here two salts dissolved in aqueous solutions react with each other to give products. Both are water…
Q: When the following net ionic equation is properly balanced, what is the coefficient for H+ in the…
A: Given chemical equation: __Cr2O72- + __H2O2 + __H+→ __Cr3+ + __O2 + __H2O
Q: Balance the following chemical equation (if necessary): Ca(C2H3O2)2(aq) + Na:CO:(aq) – CaCOs(s)'+…
A: Balanced chemical equation will be-
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Aqueous solutions of sodium carbonate reacts with calcium nitrate to form solution of sodium nitrate…
Q: Complete (where necessary) and balance the following equation. Write the stoichiometric equation,…
A: Given, __ CuSO4 (aq) + __ Zn (s) → __ Cu (s) + __________ Complete and balance the following…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: The question is based on the concept of chemical reactions. we have to complete the reaction taking…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
Q: Question is attached
A: A chemical reaction is symbolic representation of the conversion of substances to new substances. In…
Q: II. BALANCE THE CHEMICAL EQUATION BELOW. 1. Al HC1 Alci, + in producta ceactanta Pb (NO.)a + 2. Nacl…
A: A balanced chemical equation is the equation in which equal number of atoms of each element are…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: The reaction is as given below:
Q: 1. Mg (s) + HNO3 (aq) – Mg(NO3)2 (aq) + H2 (g) ,this reaction is considered to be a: * Synthesis…
A: A synthesis reaction is defined as a chemical reaction where two small chemical species react in…
Q: Complete the balanced molecular chemical equation. If not reaction occurs, write NR Fe(NO3)(aq) +…
A: Colorless sodium hydroxide (NaOH) solution is added to dark yellow iron(III) nitrate (Fe(NO3)3)…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: In this question, we will write the complete and balanced equation. You can see below.
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A:
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: The given reaction is a double-displacement reaction, which is defined as a type of chemical…
Q: MgCl2 + Fe(NO3)3 Balanced equation: Net ionic:
A: The balanced equation and net ionic equation for the above equation is given below
Q: How to balance this chemical equation? Cl2O7 + Ca(OH)2(aq) -----> Ca(ClO4)2(aq) + H2O
A: The number of atoms on reactants and products have to be same in order to balance a chemical…
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A:
Q: Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs,…
A: A balanced chemical reaction is one that has equal atoms of each element on reactants as well as…
Q: Balanced this equation. Cu + HNO3 > Cu (NO3)2 + NO2 + H2O
A: In a balanced chemical equation, all the reactants and products are written with their…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Sodium bicarbonate is often used as an antacid to neutralizeexcess hydrochloric acid in an upset stomach. Howmuch hydrochloric acid in grams can be neutralized by3.5 g of sodium bicarbonate? (Hint: Begin by writing a balancedequation for the reaction between aqueous sodiumbicarbonate and aqueous hydrochloric acid.)Write the balanced neutralization reaction that occurs between H2SO4H2SO4 and KOHKOH in aqueous solution. Phases are optional. Suppose 0.950 L0.950 L of 0.400 M H2SO40.400 M H2SO4 is mixed with 0.900 L0.900 L of 0.300 M KOH0.300 M KOH. What concentration of sulfuric acid remains after neutralization?A 20 mL volume of 0.015 M KIO3 containing an excess of KI, is added to a 0.312 g sample of a Real Lemon solution containing vitamin C. The Yellow-brown solution, caused by excess I2 is titrated to a colorless starch endpoint with 11.3 mL pf 0.106M Na2S2O3. Question: Calculate the moles and grams of vitamin C ( C6H8O6*176.06 g/mol) in the sample
- Background-info: https://drive.google.com/file/d/1G7sPTuESIgWk9wpRdqAnFcsBmOlbBfLv/view?usp=sharing Question: In carrying out Part 1 of this experiment, assume an exces of HCI(aq) reacts with 0.296 g of Mgls). If the volume of the final solution is 69.0 mL, what is the stoichiometric concentration (in mol/L) of MgCl2laq) after the reaction is complete? Report your answer to the correct number of significant figures and only include the numerical answer (no units).A solution of I3- was standardized by titrating freshly dissolved arsenious oxide (As4O6, FM 395.683). The titration of 25.00 mL of a solution prepared by dissolving 0.3663 g of As4O6 in a volume of 100.0 mL required 31.77 mL of I3-. Calculate the molarity of the I3- solution. As4O6(s) + 6H2O ⇌ 4H3AsO3 H3AsO3 + I3- + H2O ⇌ H3AsO4 + 3I- + 2H+ a. 0.01657 M b. 0.02914 M c. 0.1166 M d. 0.0957 MThe active compound in Pepto-Bismol contains C, H, O, and Bi.(a) When 0.22105 g of it was burned in excess O2, 0.1422 g of bismuth(III) oxide, 0.1880 g of carbon dioxide, and 0.02750 g of water were formed. What is the empirical formula of this compound?(b) Given a molar mass of 1086 g/mol, determine the molecular formula. (c) Complete and balance the acid-base reaction between bismuth(III) hydroxide and salicylic acid (HC7H5O3), which is used to form this compound.(d) A dose of Pepto-Bismol contains 0.600 mg of active ingredient. If the yield of the reaction in part (c) is 88.0%, what mass (in mg) of bismuth(III) hydroxide is required to prepare one dose?
- Complete the balanced dissociation equation for the compound below in aqueous solution. If the compound does not dissociate, write NR after the reaction arrow. CH3OH(aq) =?Complete the following equations to show the products that form in each of the reactions we observed in the demonstration. Include states and balance in each equation. Ca + H2O --> Li + H2O --> Na + H2O --> K + H2O -->The organic base, tris-(hydroxymethyl)aminomethane (or simply TRIS or THAM) is an excellent primary standard. A 0.2486 g sample of the primary standard grad TRIS, (CH2OH)3CNH2 (M.M. = 121.14) was dissolved in distilled water and required 24.88 mL of a hydrochloric acid solution. Calculate the molarity of the solution
- A sample containing NaCl, NaBr, & inert material weighs 1.000 g. Excess of AgNO3 gave a whiteprecipitate consisting of only AgCl and AgBr which weighs 0.5260 g. By heating the precipitate in a currentof Cl2 gas, the AgBr (187.78 g/mol) is converted to AgCl (143.32 g/mol) and the precipitate weighs 0.4260g. Find the % NaCl (58.44 g/mol) and % NaBr (102.909 mol) in the original sample.How many moles of S2O3^2- Are needed to titrate a green river water sample containing 0.50 moles of DO?Why is excess of AgNO3 (169.87 g/mol) is not used in the solution?

