Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 33QAP: A buffer is prepared in which the ratio [ H2PO4 ]/[ HPO42 ]is 3.0. (a) What is the pH of this...
Related questions
Question
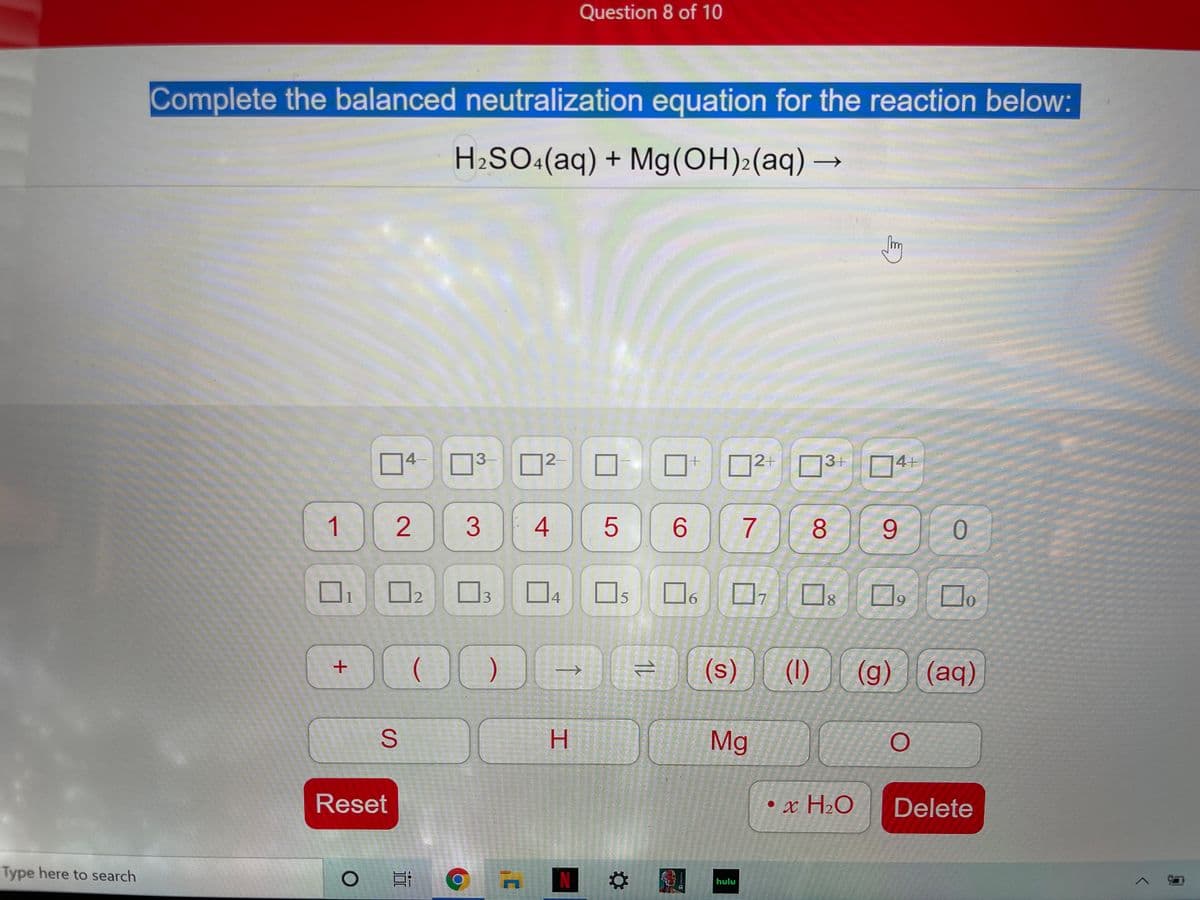
Transcribed Image Text:Question 8 of 10
Complete the balanced neutralization equation for the reaction below:
H2SO«(aq) + Mg(OH)2(aq)→
0
4-
3-
0²
2-
2+
3+
4+
2
4
6.
7
8
6.
0.
03
04
1
6.
8.
(s) |
(I)
(g) (aq)
H.
Mg
Reset
• x H2O
Delete
Type here to search
hulu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Expert Answers to Latest Homework Questions
Q: Slapshot Company makes ice hockey sticks. During the month of June, the company purchased
$125,000…
Q: ACME Company purchases for resale 1,000 widgets for $64 each. At year-end, the replacement
cost is…
Q: During May, Jimmy spent $700 to buy 20 products and sold 4 of them for $60 each. Jimmy should
record…
Q: Question:
Ace Hardware Store sells two product categories, tools and paint products. Information…
Q: please give me answer in relatable
Q: Draw the structure of the major product formed by the reaction below:
+
2 CH3NH2
Q: PROBLEM
SET C
1. IN THE SIMPLY SUPPORAD DEM SHOWN BELOW, DETERMINE THE VENICAL MOVEMENT
OF POINT C.…
Q: PROBLEM SET. 8
BEAM
1. IN THE SIMPLY SUPPORTED, SHOWN BELOW, DETERMINE THE VERTICAL MOVEMENT
OF…
Q: 2.7 The "divide and average" method, an old-time method for
approximating the square root of any…
Q: DRIPS. Western Railroad has a dividend reinvestment plan for shareholders. From 2010 to 2014, the…
Q: 2.7 The "divide and average" method, an old-time method for
approximating the square root of any…
Q: please answer in text form with proper workings and explanation for each and every part and steps…
Q: Determine the accumulated value after 5 years of deposits of $302.00 made at the beginning of every…
Q: you are working as a financial adviser either in your own business or as part of a larger…
Q: Find the SPE of the following game. (Note: the game is
the same as before except that this has no…
Q: (e) Find x₁ and x2 such that the middle area for this uniform distribution x₁ and x2 is 0.8,
that is…
Q: Problem 5-9A (Algo)
Required:
Using the percentage method for manual payroll with W-4s from 2020 or…
Q: A financial institution has the following portfolio of over-the-counter options on GBP (sterling, UK…
Q: Homework #4 Investments
Question 1 of 10
View Policies
Current Attempt in Progress
-/10 ==
You need…
Q: QUESTION
Time
Cash Flow
Period 0
-50,000.00
Period 1
15,000.00
Period 2
20,000.00
Period…
Q: Communication Systems and Procedures
State which of the following answers is correct in relation to…