Complete the following hands-on activity involving the construction of the traditional unit cells of the simple cubic, body centered cubic, and face centered cubic Bravais lattice types. 1) Please create models of simple cubic, FCC, and BCC crystal structures using a common uniform spherical objects that can be easily cut such as styrofoam balls (available from craft stores such as Michaels or Hobby Lobby or even round fruit. You can use toothpicks or low temperature hot glue to hold the parts together. For each structure (simple cubic, body centered cubic, or face-centered cubic), include a picture of the dismantled unit cell to demonstrate the number of atoms/unit cell and a picture of your unit cell. The corners of all the unit cells should consist of 1/8 spheres. 2) Verify the relationships between the lattice parameter and atomic radii shown in the lecture slides for each unit cell using the table below. Write a conclusion based on your results.
Complete the following hands-on activity involving the construction of the traditional unit cells of the simple cubic, body centered cubic, and face centered cubic Bravais lattice types. 1) Please create models of simple cubic, FCC, and BCC crystal structures using a common uniform spherical objects that can be easily cut such as styrofoam balls (available from craft stores such as Michaels or Hobby Lobby or even round fruit. You can use toothpicks or low temperature hot glue to hold the parts together. For each structure (simple cubic, body centered cubic, or face-centered cubic), include a picture of the dismantled unit cell to demonstrate the number of atoms/unit cell and a picture of your unit cell. The corners of all the unit cells should consist of 1/8 spheres. 2) Verify the relationships between the lattice parameter and atomic radii shown in the lecture slides for each unit cell using the table below. Write a conclusion based on your results.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Instead of creating one, just draw the model on paper, be specific and show work! This is for materials of engineering class!
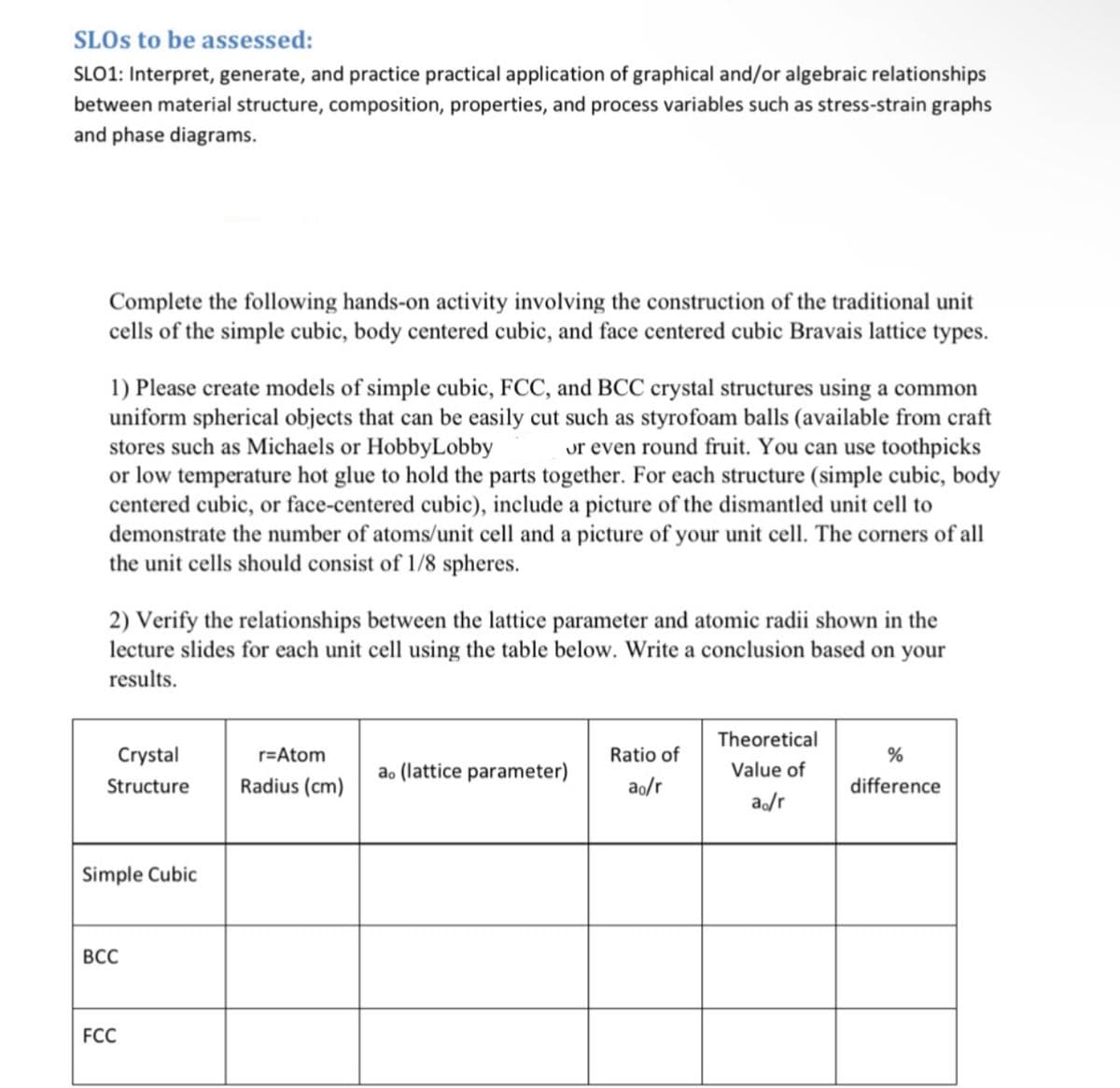
Transcribed Image Text:SLOS to be assessed:
SLO1: Interpret, generate, and practice practical application of graphical and/or algebraic relationships
between material structure, composition, properties, and process variables such as stress-strain graphs
and phase diagrams.
Complete the following hands-on activity involving the construction of the traditional unit
cells of the simple cubic, body centered cubic, and face centered cubic Bravais lattice types.
1) Please create models of simple cubic, FCC, and BCC crystal structures using a common
uniform spherical objects that can be easily cut such as styrofoam balls (available from craft
stores such as Michaels or Hobby Lobby or even round fruit. You can use toothpicks
or low temperature hot glue to hold the parts together. For each structure (simple cubic, body
centered cubic, or face-centered cubic), include a picture of the dismantled unit cell to
demonstrate the number of atoms/unit cell and a picture of your unit cell. The corners of all
the unit cells should consist of 1/8 spheres.
2) Verify the relationships between the lattice parameter and atomic radii shown in the
lecture slides for each unit cell using the table below. Write a conclusion based on your
results.
Crystal
Structure
Simple Cubic
BCC
FCC
r=Atom
Radius (cm)
ao (lattice parameter)
Ratio of
ao/r
Theoretical
Value of
ao/r
%
difference
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY