Complete the following nuclear bombardment equation by filling in the nuclear symbol for the missing species. U + ôn Cs + 2 ôn Is this a nuclear fusion reaction or a nuclear fission reaction? (
Complete the following nuclear bombardment equation by filling in the nuclear symbol for the missing species. U + ôn Cs + 2 ôn Is this a nuclear fusion reaction or a nuclear fission reaction? (
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter14: Nuclear Chemistry
Section: Chapter Questions
Problem 14.97PAE
Related questions
Question
Transcribed Image Text:TUTOR
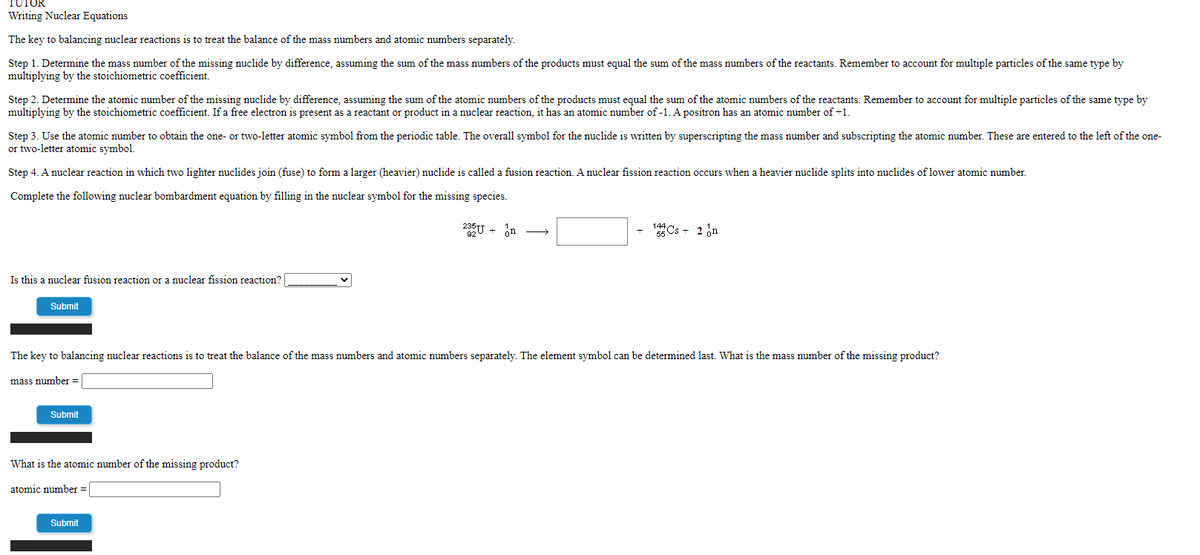
Writing Nuclear Equations
The key to balancing nuclear reactions is to treat the balance of the mass numbers and atomic numbers separately.
Step 1. Determine the mass number of the missing nuclide by difference, assuming the sum of the mass numbers of the products must equal the sum of the mass numbers of the reactants. Remember to account for multiple particles of the same type by
multiplying by the stoichiometric coefficient.
Step 2. Determine the atomic number of the missing nuclide by difference, assuming the sum of the atomic numbers of the products must equal the sum of the atomic numbers of the reactants. Remember to account for multiple particles of the same type by
multiplying by the stoichiometric coefficient. If a free electron is present as a reactant or product in a nuclear reaction, it has an atomic number of -1. A positron has an atomic number of +1.
Step 3. Use the atomic number to obtain the one- or two-letter atomic symbol from the periodic table. The overall symbol for the nuclide is written by superscripting the mass number and subscripting the atomic number. These are entered to the left of the one-
or two-letter atomic symbol.
Step 4. A nuclear reaction in which two lighter nuclides join (fuse) to form a larger (heavier) nuclide is called a fusion reaction. A nuclear fission reaction occurs when a heavier nuclide splits into nuclides of lower atomic number.
Complete the following nuclear bombardment equation by filling in the nuclear symbol for the missing species.
235U + in
Cs + 2 in
Is this a nuclear fusion reaction or a nuclear fission reaction?
Submit
The key to balancing nuclear reactions is to treat the balance of the mass numbers and atomic numbers separately. The element symbol can be determined last. What is the mass number of the missing product?
mass number =
Submit
What is the atomic number of the missing product?
atomic number =
Submit
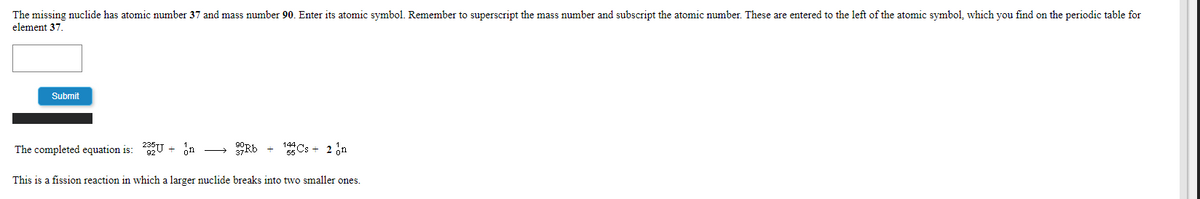
Transcribed Image Text:The missing nuclide has atomic number 37 and mass number 90. Enter its atomic symbol. Remember to superscript the mass number and subscript the atomic number. These are entered to the left of the atomic symbol, which you find on the periodic table for
element 37.
Submit
The completed equation is: U + n
Rb
5 Cs + 2 on
This is a fission reaction in which a larger nuclide breaks into two smaller ones.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning