Complete the following reactions. Show the stereother DMF 411B+ + Na CN हुई CHS сиз сиз-енен СЊCRO С.Ненз Br Ea +C12/10 Perotidi NQ OH > MgBr ol. + HBr + pec + pels 6 CH-CH= снесия CH3 NBS +H2O heat H₂ crown NANH2 ничко ссия H₂ 504> Cl Си-ен- сна-ен-он Кмпой (сд re
Complete the following reactions. Show the stereother DMF 411B+ + Na CN हुई CHS сиз сиз-енен СЊCRO С.Ненз Br Ea +C12/10 Perotidi NQ OH > MgBr ol. + HBr + pec + pels 6 CH-CH= снесия CH3 NBS +H2O heat H₂ crown NANH2 ничко ссия H₂ 504> Cl Си-ен- сна-ен-он Кмпой (сд re
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter10: Oxidation And Reduction
Section: Chapter Questions
Problem 4CTQ
Related questions
Question
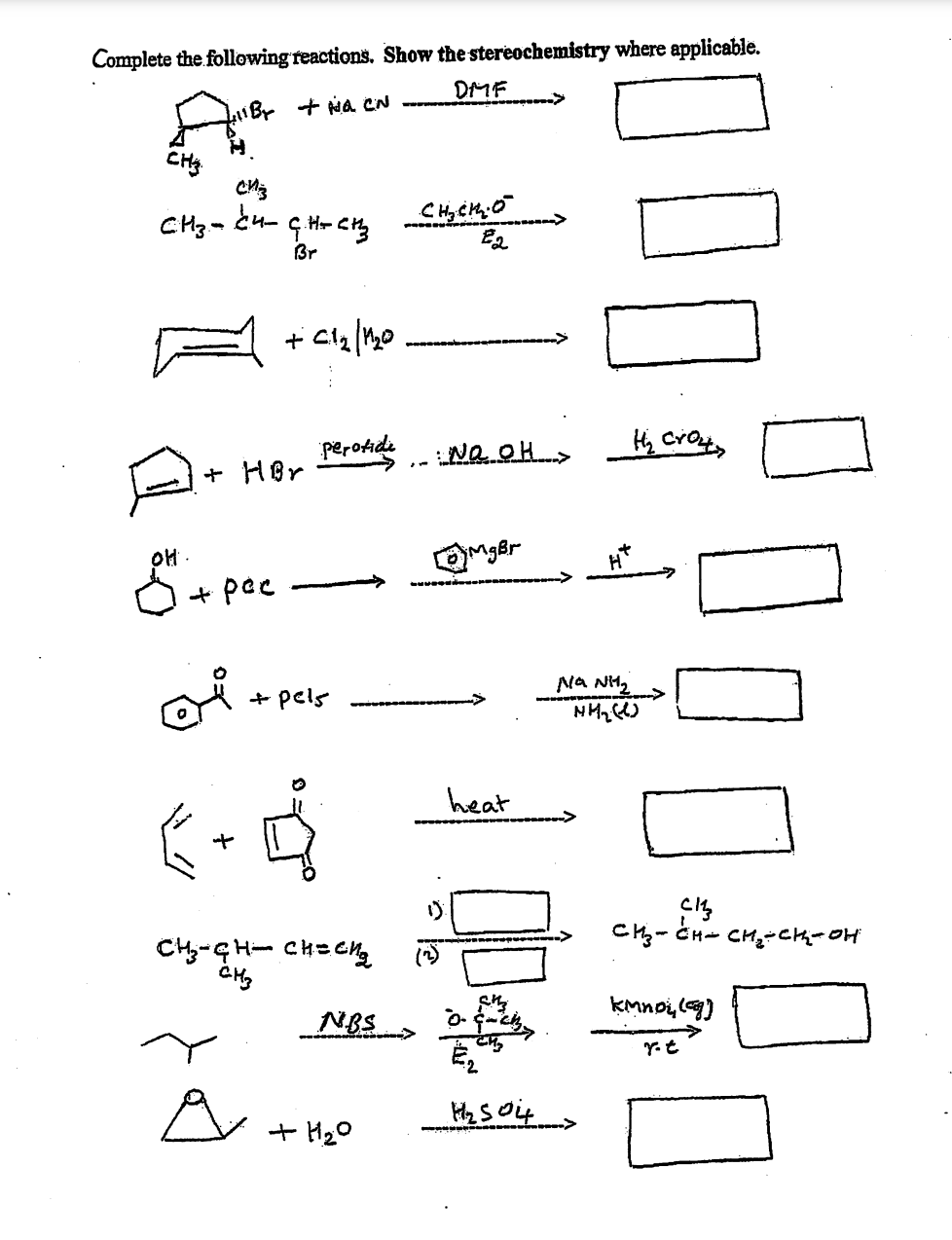
Transcribed Image Text:Complete the following reactions. Show the stereochemistry where applicable.
DMF
iBT + H₂ CN
CHS
сиз
си-ей антена сцена в
E2
Br
+cla/mo
perotide
: No OH >
H₂ Croutz
MgBr
애
+ HBr
+ pac
po
ой
+ pels
❤
CH-CH- снесия
стану
NBS
+H20
heat
H
NANH2
HH₂(1)
H₂ 504 >
Cl
Си-Ен= CH2-CH-он
Кмпой (G)
YL
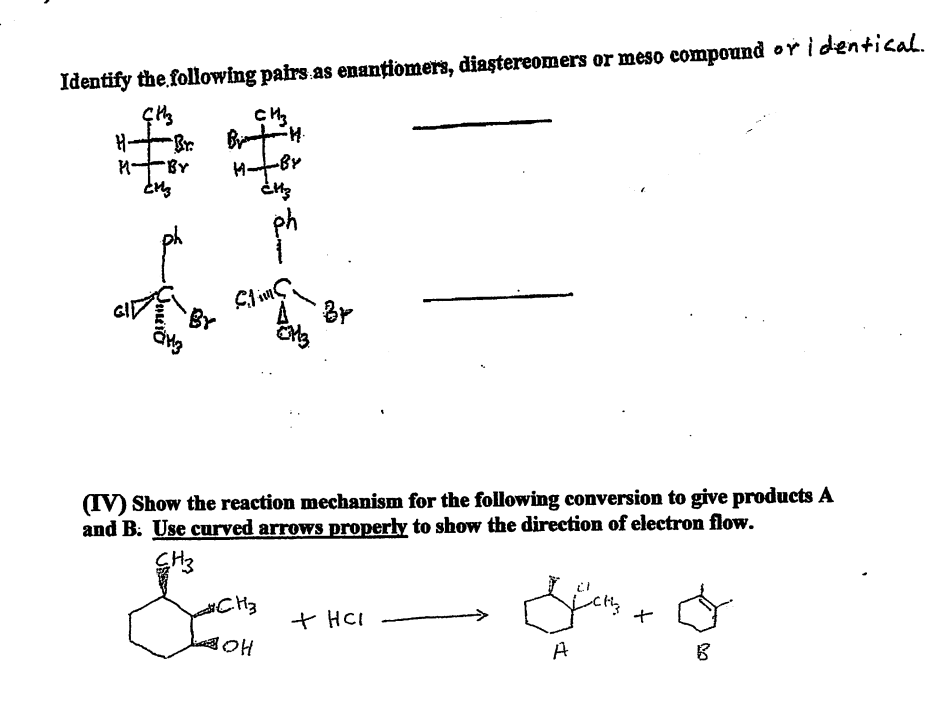
Transcribed Image Text:Identify the following pairs as enantiomers, diastereomers or meso compound or identical.
ÇHz
CH3
H-
Brim
M
Br
(IV) Show the reaction mechanism for the following conversion to give products A
and B. Use curved arrows properly to show the direction of electron flow.
CH3
#CH3
Sans + O
+ HCI
BOH
A
B
-BY
CM3
-H.
-BY
HB
¿H3
Slims
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
This last part didnt post! But I was wondering, for your #6, how do both cls leave?
![Answer the following:
R₁
·X + Nu-
I[=+13.5⁰
II product with [x]=-8.4°
In the above reaction, optically active compound I has a specific rotation of +13.5°, and
compound II with a 100% enantiomeric excess exists with a specific rotation of +16.8° or
-16.8°. However, when compound II was prepared from I the specific rotation of
the product was - 8.4º,
a) Explain this observation in terms of the mechanism of the reaction. (Show the reaction
Mechanism)
b) Calculate the enantiomeric excees of the product
c) What is the percent composition of the product.
R₂-C-Nu
R3
Where: R₁ R2 R³ ‡X #Nu](https://content.bartleby.com/qna-images/question/c7779c51-24b0-42c0-9e0b-df6f8cdc5c96/8124d474-3cbd-41ee-98f2-6aa2242226a5/a4s7qom_thumbnail.png)
Transcribed Image Text:Answer the following:
R₁
·X + Nu-
I[=+13.5⁰
II product with [x]=-8.4°
In the above reaction, optically active compound I has a specific rotation of +13.5°, and
compound II with a 100% enantiomeric excess exists with a specific rotation of +16.8° or
-16.8°. However, when compound II was prepared from I the specific rotation of
the product was - 8.4º,
a) Explain this observation in terms of the mechanism of the reaction. (Show the reaction
Mechanism)
b) Calculate the enantiomeric excees of the product
c) What is the percent composition of the product.
R₂-C-Nu
R3
Where: R₁ R2 R³ ‡X #Nu
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

